Session Information
The 2020 Pediatric Rheumatology Symposium, originally scheduled for April 29 – May 2, was postponed due to COVID-19; therefore, abstracts were not presented as scheduled.
Session Type: Poster Breakout Session
Session Time: 5:10PM-5:40PM
Background/Purpose: Despite efforts to identify biomarkers to guide therapy in the treatment of juvenile idiopathic arthritis (JIA), drug therapy remains a trial-and-error process. In the case of methotrexate (MTX) there has been a variety of approaches employed in the identification of molecular markers of drug response in JIA that have primarily focused on the targeted measurement of drug levels or biomolecules believed to be associated with the pharmacological activity of MTX. In contrast to previous work, this study utilizes a global metabolomics strategy to identify biochemical pathways and metabolomic markers associated with MTX response in the treatment of JIA.
Methods: Plasma samples analyzed in this study were from an IRB-approved single center prospective cohort study of biomarkers of MTX response in patients with JIA and included a cohort of 30 JIA patients initiated on MTX at a dose of 15 mg/m2. 100% of patients met the ACR classification criteria for JIA. Clinical response to MTX was determined based on the ACR Pedi 70 response criteria and non-response was determined by failing to meet ACR Pedi 30 response criteria. Changes in the plasma metabolomic profiles were determined by the comparison of metabolite levels immediately prior to the initiation of MTX and after 3-months of MTX therapy based on normalized peak intensities. A global metabolomics approach capable of detecting over 800 known metabolites was conducted utilizing three independent metabolomic profiling platforms at the NIH West Coast Metabolomics Center at UC-Davis. The resulting metabolomic data was evaluated by univariate and multivariate analysis using MetaboAnalyst 3.0. Metabolic enrichment analyses were conducted using ChemRICH to identify metabolic pathways associated with MTX activity. Association of changes in metabolites with clinical response was assessed by Wilcoxon rank-sum analysis.
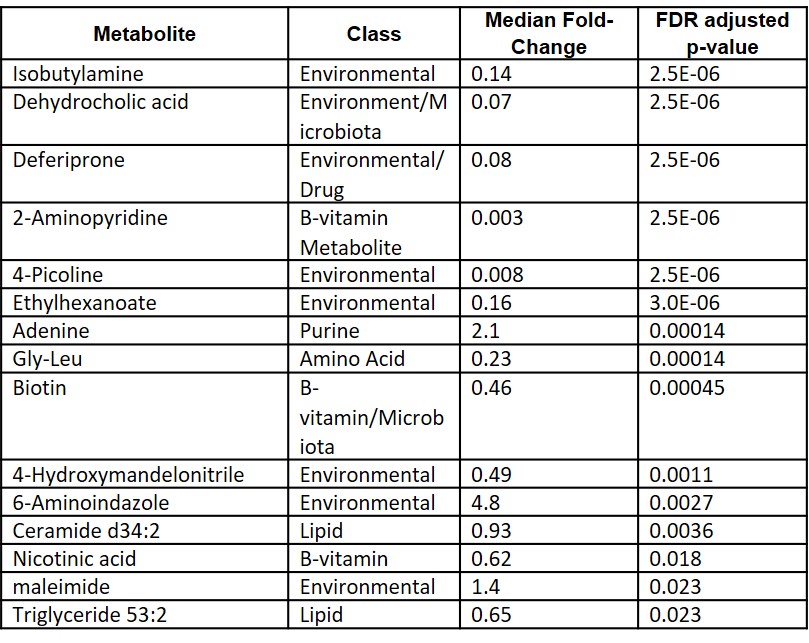
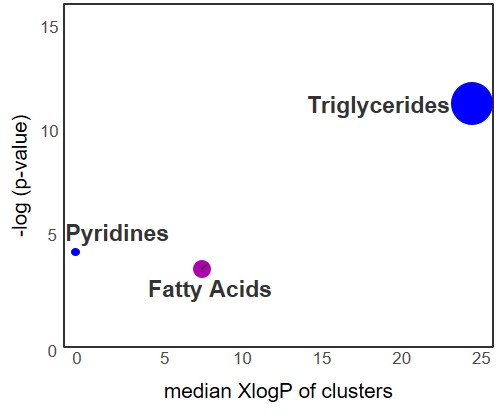
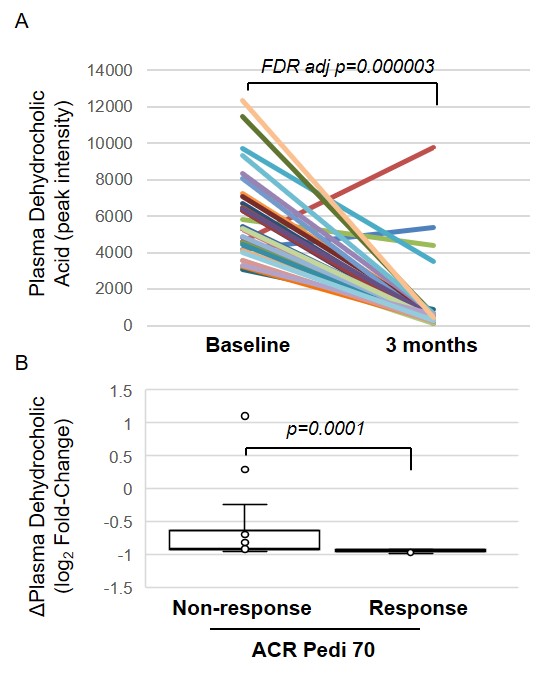
Results: Among the 673 identified metabolites detected in the plasma, 15 metabolites were found to be significantly altered following the initiation of MTX (Table 1). Identified metabolites included metabolites associated with exogenous environmental exposures (e.g. isobutylamine), gut microbial metabolism (e.g. dehydrocholic acid), B vitamin metabolism (e.g. nicotinic acid) and purine metabolism (i.e. adenine). Chemical enrichment analysis supported significant changes in plasma triglycerides, fatty acids, and pyridines following the initiation of MTX (Figure 1). Of the metabolites found to be significantly altered by MTX therapy, only reductions in plasma dehydrocholic acid levels were found to be associated with the achievement of ACR Pedi 70 response criteria at 3-months (Figure 3).
Conclusion: This work demonstrates that initiation of MTX therapy is associated with broad alterations in the plasma metabolome of patients with JIA and identifies dehydrocholic acid as a potential biomarker of MTX response in JIA. This global approach has also identified novel pathways associated with drug response for future exploration.
To cite this abstract in AMA style:
Funk R, Becker M. Association of Plasma Metabolomic Profiles with Methotrexate Response in Juvenile Idiopathic Arthritis [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 4). https://acrabstracts.org/abstract/association-of-plasma-metabolomic-profiles-with-methotrexate-response-in-juvenile-idiopathic-arthritis/. Accessed .« Back to 2020 Pediatric Rheumatology Symposium
ACR Meeting Abstracts - https://acrabstracts.org/abstract/association-of-plasma-metabolomic-profiles-with-methotrexate-response-in-juvenile-idiopathic-arthritis/