Session Information
Session Type: Abstract Session
Session Time: 2:15PM-2:30PM
Background/Purpose: Prior studies have evaluated the efficacy of antiviral medications on acute outcomes of COVID-19 infection as well as the risk of post-acute sequelae of COVID-19 (PASC, i.e., “long COVID”) in the general population. However, there are little data regarding the effectiveness of these agents for reducing the risk of PASC in individuals with systemic autoimmune rheumatic diseases (SARDs).
Methods: We consecutively enrolled individuals with SARDs into the prospective RheumCARD study at a large US healthcare system following their COVID-19 infection from December 16, 2021 (following the start of the Omicron era and availability of oral outpatient therapy) until January 30, 2024. Individuals who were hospitalized for acute COVID-19 and/or who received COVID-19 monoclonal antibodies or remdesivir were excluded. Individuals completed surveys at least 28 days following COVID-19 infection regarding the presence vs. absence of persistent symptoms as well as the duration of symptoms. We assessed the odds of PASC at both 28 and 90 days following COVID-19 infection and used multivariable logistic regression to compare those who did versus did not receive oral outpatient antiviral medication (either nirmatrelvir/ritonavir or molnupiravir) for acute COVID-19 infection. The multivariable model was adjusted for age, sex, and race.
Results: Among 430 patients (49% treated with nirmatrelvir/ritonavir or molnupiravir and 51% not treated with antiviral medications), the mean age at COVID-19 onset was 56.2 years, 82% were female, and 91% were White (Table). The most common SARD category was inflammatory arthritis (37%), followed by connective tissue disease (11%). Many were using conventional synthetic DMARDs (49%), biologic DMARDs (41%); 7% were using CD20 inhibitors) and 15% were using systemic glucocorticoids at the time of COVID-19 infection. Nearly all (97%) were vaccinated at the time of COVID-19 infection. The prevalence of PASC at 28 days in those who did vs. did not receive antiviral therapy was 33% vs. 31% (p=0.77). However, the prevalence of PASC at 90 days in those who did vs. did not receive antiviral therapy was 5% vs. 11% (p=0.02). Compared to those who did not receive antiviral medications (n=221), those prescribed antiviral therapy (n=209) had significantly lower odds of PASC at 90 days (adjusted OR 0.42, 95% CI: 0.18-0.95), while there was no difference in odds of PASC between groups at 28 days (Figure).
Conclusion: In this prospective contemporary cohort, individuals with SARDs who received oral outpatient antiviral therapy had significantly lower odds of PASC lasting at least 90 days following COVID-19 infection than those who did not receive antiviral therapy, while odds of PASC lasting at least 28 days did not differ between groups. These findings support additional potential benefits of oral outpatient antiviral treatment for PASC risk among individuals with SARDs who are eligible and do not have contraindications.
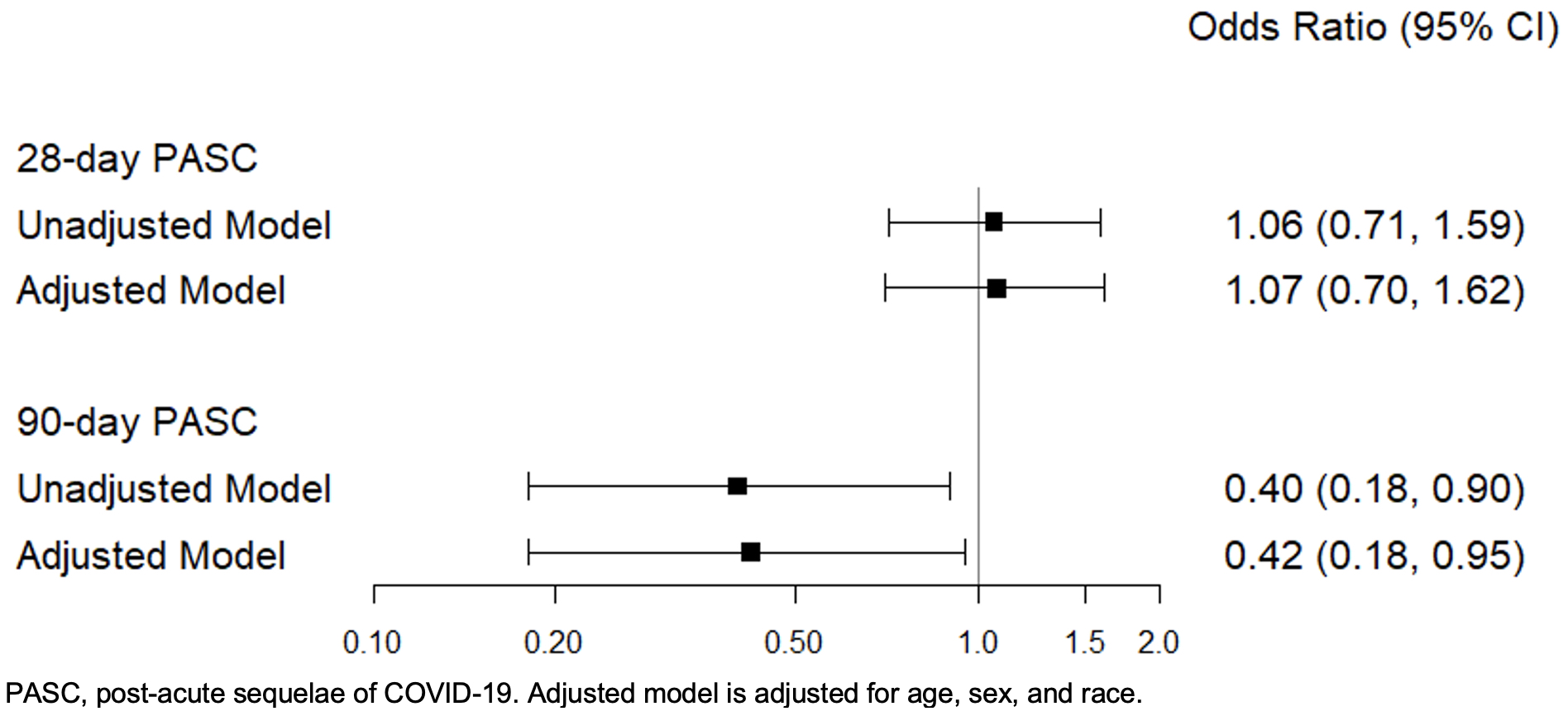 Table 1: Baseline characteristics in individuals with rheumatic disease and COVID-19 who did vs. did not receive oral outpatient antiviral therapy for acute COVID-19 infection.
Table 1: Baseline characteristics in individuals with rheumatic disease and COVID-19 who did vs. did not receive oral outpatient antiviral therapy for acute COVID-19 infection.
.jpg) Figure. Odds of PASC at 28 and 90 days in individuals with rheumatic diseases and COVID-19 who did vs. did not receive antiviral therapy for acute COVID-19 infection
Figure. Odds of PASC at 28 and 90 days in individuals with rheumatic diseases and COVID-19 who did vs. did not receive antiviral therapy for acute COVID-19 infection
To cite this abstract in AMA style:
Negron M, Wang J, Wang X, O'Keeffe L, Qian G, Mueller K, Saavedra A, Davis N, Getachew L, Sparks J, Patel N. Association of Oral Outpatient Antiviral Medications for COVID-19 with the Risk of Post-Acute Sequelae of COVID-19 in Individuals with Systemic Autoimmune Rheumatic Diseases [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/association-of-oral-outpatient-antiviral-medications-for-covid-19-with-the-risk-of-post-acute-sequelae-of-covid-19-in-individuals-with-systemic-autoimmune-rheumatic-diseases/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/association-of-oral-outpatient-antiviral-medications-for-covid-19-with-the-risk-of-post-acute-sequelae-of-covid-19-in-individuals-with-systemic-autoimmune-rheumatic-diseases/
