Session Information
Date: Monday, November 9, 2020
Title: RA – Diagnosis, Manifestations, & Outcomes Poster IV: Lifespan of a Disease
Session Type: Poster Session D
Session Time: 9:00AM-11:00AM
Background/Purpose: Obesity affects 30-40% of RA patients and is associated with higher clinical disease activity measures and progressive disability. Studies suggest that obesity may be associated with poor response to TNF inhibitors (TNFi), but the degree to which these prior results were impacted by unmeasured confounding factors is unclear. We aimed to evaluate differences in response to MTX and TNFi across BMI categories among RA patients in a real-world setting, accounting for multiple confounding factors.
Methods: We conducted a retrospective cohort study within the Veterans Affairs RA registry. Adult patients initiating a course of MTX or TNFi (with no use in prior 90 days) who had at least moderate disease activity (DAS28 >3.2) at baseline and one or more clinical visit with DAS28 30 days to 6 months later were included. BMI (kg/m2) was categorized as underweight (< 20), normal (20-25), overweight (25-30), class 1 obesity (30-35) and class 2-3 obesity ( >35). Response was defined as achieving at least low disease activity (LDA; DAS28 < 3.2) or the minimally clinically important difference (MCID) in DAS28 ( >=1.2) within 6 months. We also extracted disease duration, Multi-Dimensional HAQ score, smoking status, and extra-articular features of disease from the database. Comorbidities were obtained from medical record databases within 1-year prior to initiation. Anti−CCP antibody and RF were measured in banked serum obtained at the time of enrolment. Concurrent RA medications, including prior courses of conventional DMARDs and biologic therapies were determined from VA pharmacy data. The association between BMI and response to treatment within 6 months was estimated in logistic regression models adjusted for demographics, calendar year, RA severity factors, prior/concomitant therapies, and comorbidities at baseline.
Results: We identified 738 eligible MTX courses (471 unique patients) and 1165 TNFi courses (509 unique patients). In both cohorts, the population was primarily male (90% in MTX, 92% in TNFi), and two-thirds were either overweight or obese (73% in MTX, 70% in TNFi). Median baseline DAS28 score was higher among severely obese MTX initiators. Other characteristics are depicted in Table 1. The overall unadjusted response was achieved in 45% of MTX courses and 52% of TNFi courses. While the response rates were numerically lowest among underweight patients in TNFi cohorts before and after adjustment, responses were not associated with BMI categories in either cohort with or without adjustment (all p >0.10). In adjusted models, the odds of clinical response for all outcomes were similar across BMI categories in both cohorts (Table 2; Fig 1).
Conclusion: In this predominantly male veteran population, clinical response following the initiation of MTX or TNFi for RA in a real-world registry data did not meaningfully differ by BMI categories after controlling for a robust set of covariates. These findings argue against a biological basis for differences in treatment response in RA patients with higher BMI. Future head to head comparison studies among obese patients may be necessary to determine whether a particular treatment strategy is more efficacious in this group.
 Table 1. Baseline characteristics of MTX and TNFi initiators stratified by BMI category
Table 1. Baseline characteristics of MTX and TNFi initiators stratified by BMI category
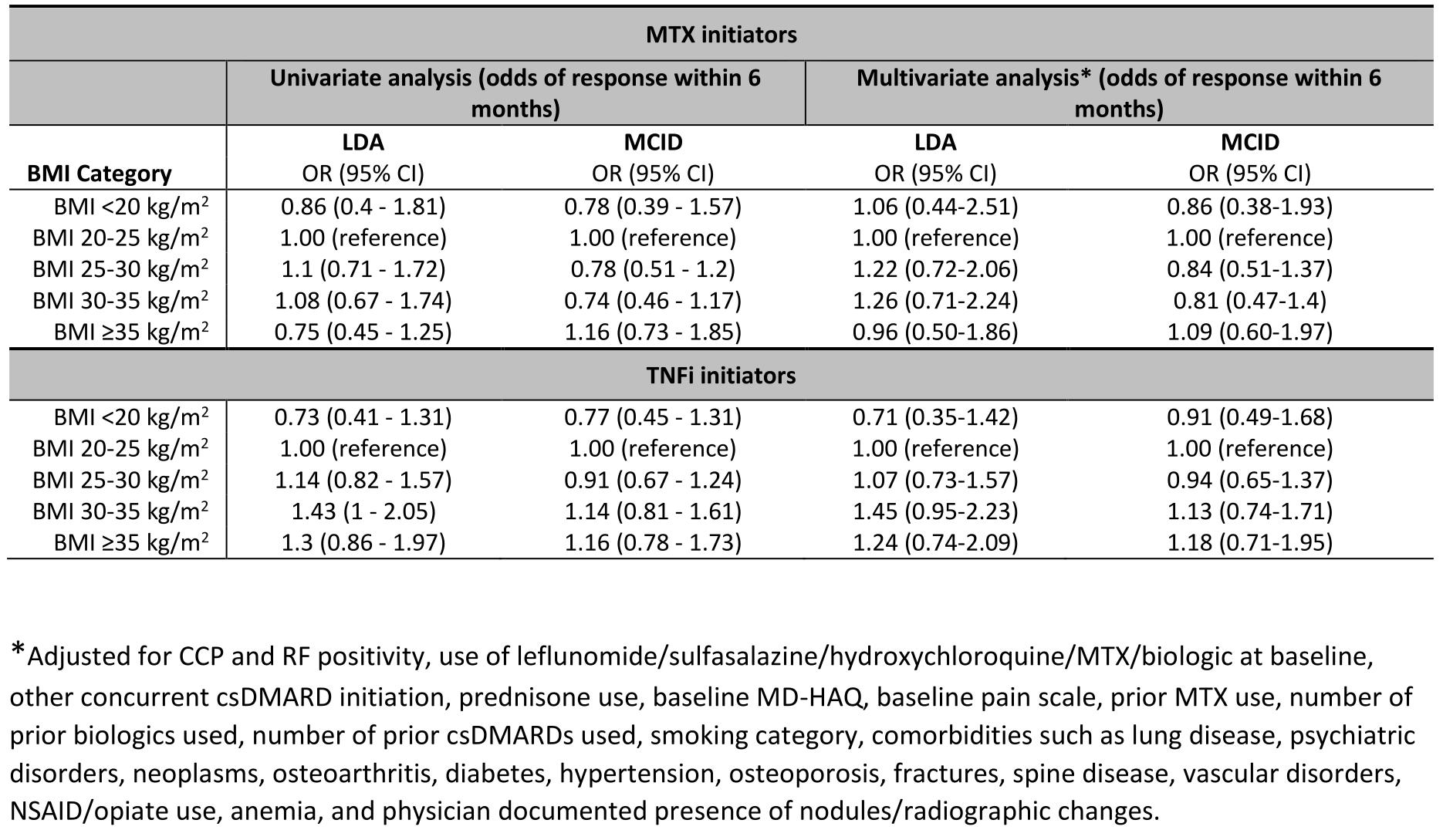 Table 2: Univariate and multivariate analyses reporting response percent and fully adjusted odds ratio, respectively, among methotrexate and tumor necrosis factor inhibitor initiators
Table 2: Univariate and multivariate analyses reporting response percent and fully adjusted odds ratio, respectively, among methotrexate and tumor necrosis factor inhibitor initiators
 Figure 1: Predicted probabilities of combined (primary) response in methotrexate and tumor necrosis factor inhibitor initiators based on logistic regression models
Figure 1: Predicted probabilities of combined (primary) response in methotrexate and tumor necrosis factor inhibitor initiators based on logistic regression models
To cite this abstract in AMA style:
Poudel D, Mikuls T, George M, England B, Cannon G, Sauer B, Baker J. Association of Obesity with Treatment Response to Methotrexate or Tumor Necrosis Factor Inhibitors in Patients with Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/association-of-obesity-with-treatment-response-to-methotrexate-or-tumor-necrosis-factor-inhibitors-in-patients-with-rheumatoid-arthritis/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/association-of-obesity-with-treatment-response-to-methotrexate-or-tumor-necrosis-factor-inhibitors-in-patients-with-rheumatoid-arthritis/
