Session Information
Date: Monday, October 27, 2025
Title: (1553–1591) Systemic Sclerosis & Related Disorders – Clinical Poster II
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Soluble CD13 (sCD13), released by the cleavage of cell surface CD13 by matrix metalloproteinase-14 (MMP14), has potent proinflammatory, angiogenic, and arthritogenic activities. The receptors for sCD13 are bradykinin receptor B1 (B1R) and protease-activated receptor 4 (PAR4). Building on our recent study showing that the sCD13-B1R axis was critically involved in promoting fibrosis in systemic sclerosis (SSc)1, we hypothesized that plasma sCD13 levels are associated with SSc clinical features. This study aimed to measure plasma sCD13 levels in SSc patients and examine their correlations with disease phenotype, vascular complications, skin and lung fibrosis, and other clinical characteristics. The expression of genes associated with the sCD13-B1R pathway was also examined in peripheral blood cells.
Methods: Plasma sCD13 was measured using ELISA. SSc patients and healthy controls were recruited at the University of Michigan Scleroderma Program. Three patient cohorts were used to evaluate sCD13 levels for disease subtypes, vascular involvement, and early disease. Clinical data was recorded. Vascular complications were defined as a history of at least one of the following: digital ulcers, pulmonary arterial hypertension, or SSc renal crisis. Transcriptomic data from the GEO dataset GSE181228 was extracted to evaluate genes relevant to CD13 in peripheral blood cells. Statistical analyses were performed using t-tests, chi-square tests, and correlation analysis, and p< 0.05 was considered significant.
Results: Plasma sCD13 levels were significantly higher in diffuse cutaneous (dc)SSc patients compared to limited cutaneous SSc patients and healthy controls. There was no significant association between vascular complications and plasma sCD13 levels in SSc patients with vascular complications compared to those without. Among early-stage SSc patients (diagnosed within 3 years of disease onset), there was no significant difference in mRSS between the high and low sCD13 groups (Table 1). However, the change in mRSS at one-year follow-up showed a significant decrease in the high sCD13 patient group (Figure 1). The transcriptomic analysis showed that ANPEP (gene encoding CD13), MMP14 and F2RL3 (encoding PAR4) were upregulated in SSc patients compared to healthy controls, and a positive correlation was observed between ANPEP and MMP14, as well as between ANPEP and F2RL3. Furthermore, significant positive correlations were observed between ANPEP and TGFB1 or IL6, as well as between MMP14 and TGFB1 or IL6. F2RL3 also showed a significant correlation with TGFB1 (Figure 2).
Conclusion: Our study showed that the increased plasma sCD13 levels in patients with SSc did not correlate with the clinical features of the disease. Given that sCD13 is elevated in dcSSc patients and that it promotes myofibroblast transformation in SSc skin, these results suggest that tissue-level sCD13 might be more significant than systemic levels. In addition, MMP14 and CD13 expression on blood cells, potentially enhanced by TGF-β and IL-6, could influence circulating CD13 levels in SSc.1. Muraoka S et al. Targeting CD13/Aminopeptidase N as a Novel Therapeutic Approach for Scleroderma Fibrosis. Arthritis Rheumatol. 2025;77(1):80-91.
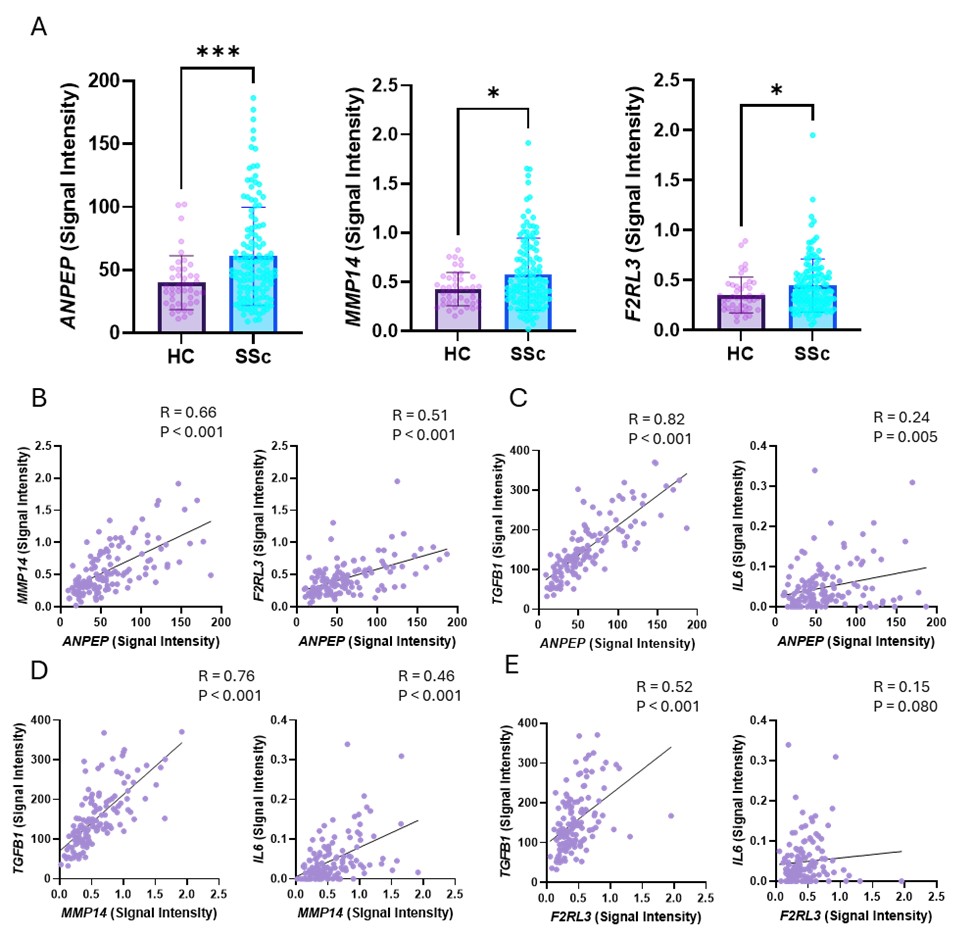 Table 1. Baseline characteristics of patients with early-onset SSc and comparison between High and Low sCD13 status
Table 1. Baseline characteristics of patients with early-onset SSc and comparison between High and Low sCD13 status
High sCD13 status was defined as plasma values exceeding 384.3 ng/mL. SSc, systemic sclerosis; sCD13 soluble CD13; SD, standard deviation; IQR, interquartile range; mRSS, modified Rodnan skin score; ILD, interstitial lung disease, PAH, pulmonary arterial hypertension; FVC, forced vital capacity; DLCO, diffusing capacity of the lung for carbon monoxide.
.jpg) Figure 1. Comparison of mRSS changes between High and Low sCD13 groups in SSc patients within 3 years of disease onset.
Figure 1. Comparison of mRSS changes between High and Low sCD13 groups in SSc patients within 3 years of disease onset.
(A) Plasma sCD13 levels were significantly higher in SSc patients within 3 years of disease onset compared to HC. (B) No significant difference in baseline mRSS was observed between the high and low sCD13 groups. (C) No significant correlation was observed between plasma sCD13 levels and baseline mRSS. (D) mRSS measured one year follow up visit showed no significant difference between the high and low sCD13 groups. (E) The change in mRSS (ΔmRSS) over one year showed a significant decrease in the high sCD13 group. (F) A significant negative correlation was observed between ΔmRSS over one year and plasma sCD13 levels. Results are expressed as mean ± SD or median [IQR]. *P < 0.05, **P < 0.01, ***P < 0.001. sCD13, soluble CD13; HC, healthy controls; SSc, systemic sclerosis; mRSS, modified Rodnan skin score; SD, standard deviation; IQR, interquartile range.
.jpg) Figure2. Evaluation of CD13, MMP14 and PAR4 expression in SSc patients and healthy controls
Figure2. Evaluation of CD13, MMP14 and PAR4 expression in SSc patients and healthy controls
(A) Expression levels of ANPEP, MMP14 and F2RL3 were significantly higher in SSc patients compared to healthy controls. (B) ANPEP expression levels were significantly and positively associated with MMP14 and F2RL3. (C) ANPEP, MMP14 and F2RL3 expression levels showed a significant correlation with TGFB1 and IL6, key cytokines involved in fibrosis and inflammation in SSc, although the correlation between F2RL3 and IL6 was not statistically significant (P = 0.08). Results are expressed as mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001. Significance was determined by (A) Mann-Whitney test and (B-C) Spearman correlation. SSc, systemic sclerosis.
To cite this abstract in AMA style:
Ikari Y, Dey P, St. Clair J, Webber A, Foster C, Chen Y, Ali R, Khanna D, Fox D, Tsou P. Association of levels of soluble CD13 with clinical features and fibrosis in systemic sclerosis patients [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/association-of-levels-of-soluble-cd13-with-clinical-features-and-fibrosis-in-systemic-sclerosis-patients/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/association-of-levels-of-soluble-cd13-with-clinical-features-and-fibrosis-in-systemic-sclerosis-patients/
