Session Information
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Ixekizumab (IXE) is a high-affinity monoclonal antibody that selectively targets interleukin-17A. In a phase 3 study, IXE was superior to placebo (PBO) in achieving ACR20 responses at Week (Wk) 24 in biologic DMARD-naïve (bDMARD-naïve) PsA patients1. The objective of this analysis was to explore the association of early skin improvement with ACR responses among IXE-treated patients with both PsA and psoriasis (Ps).
Methods: In a phase 3, multi-center, double-blind randomized trial (SPIRIT-P1; NCT01695239), 417 bDMARD-naïve patients with active PsA were randomized to receive up to 24 wks of treatment of PBO (N=106), adalimumab 40 mg (ADA; active reference arm) once every 2 wks (Q2W; N=101), or IXE 80 mg Q2W (N=103) or Q4W (N=107) following an 160 mg initial dose at Wk 0. In PsA patients with moderate-to-severe Ps (≥3% body surface area [BSA]), ACR and Psoriasis Area and Severity Index (PASI) responses were analyzed up to Wk 24. Patients were demarcated into the following categories based on percent improvement from baseline: PASI:<50, PASI:50 to <75, PASI:75 to <90, and PASI:90 to 100. Missing ACR and PASI responses were imputed using non-responder imputation (NRI) and last observation carried forward (LOCF) methods, respectively. Logistic regression model was utilized to compare ACR response across PASI improvement categories.
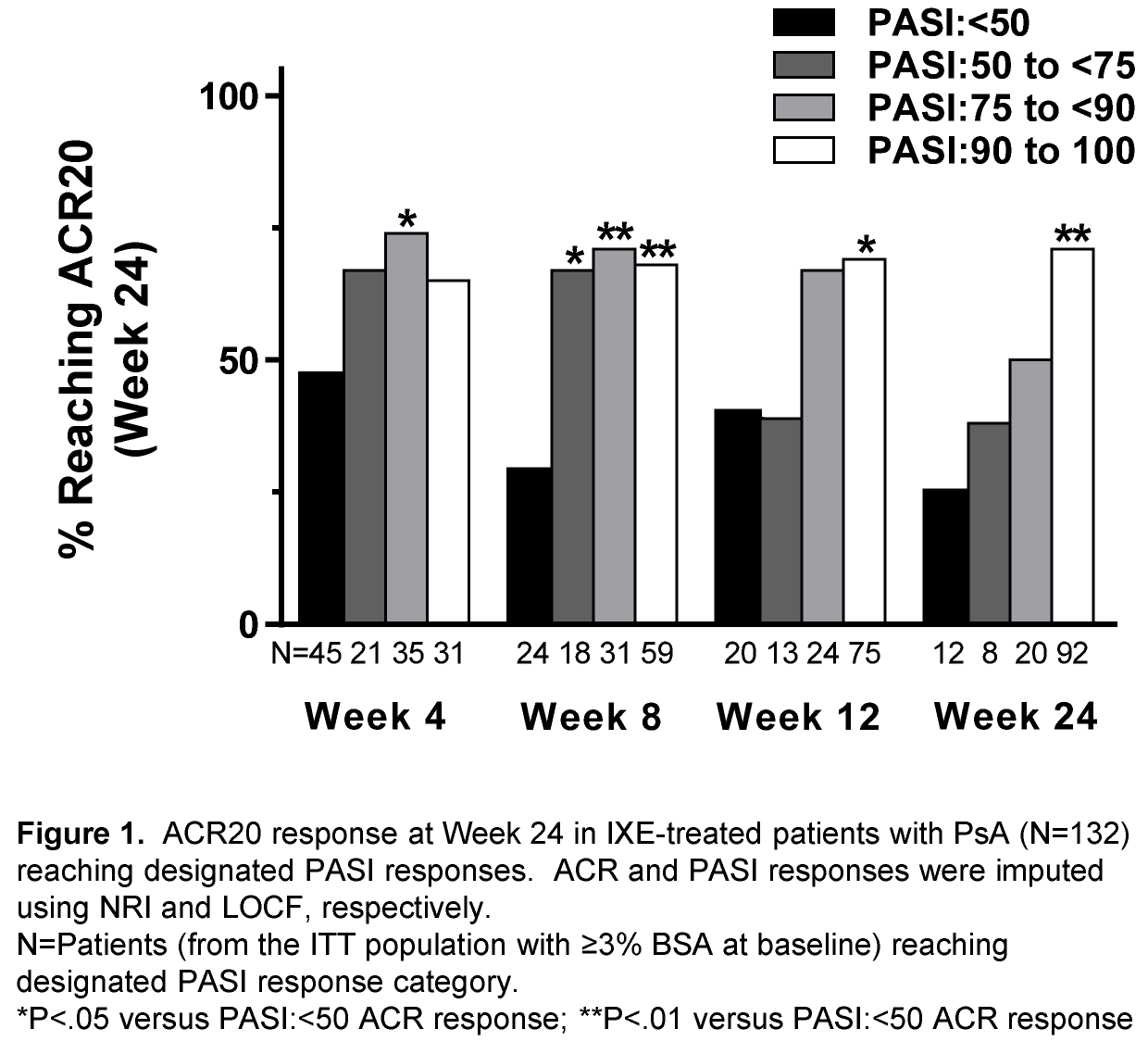
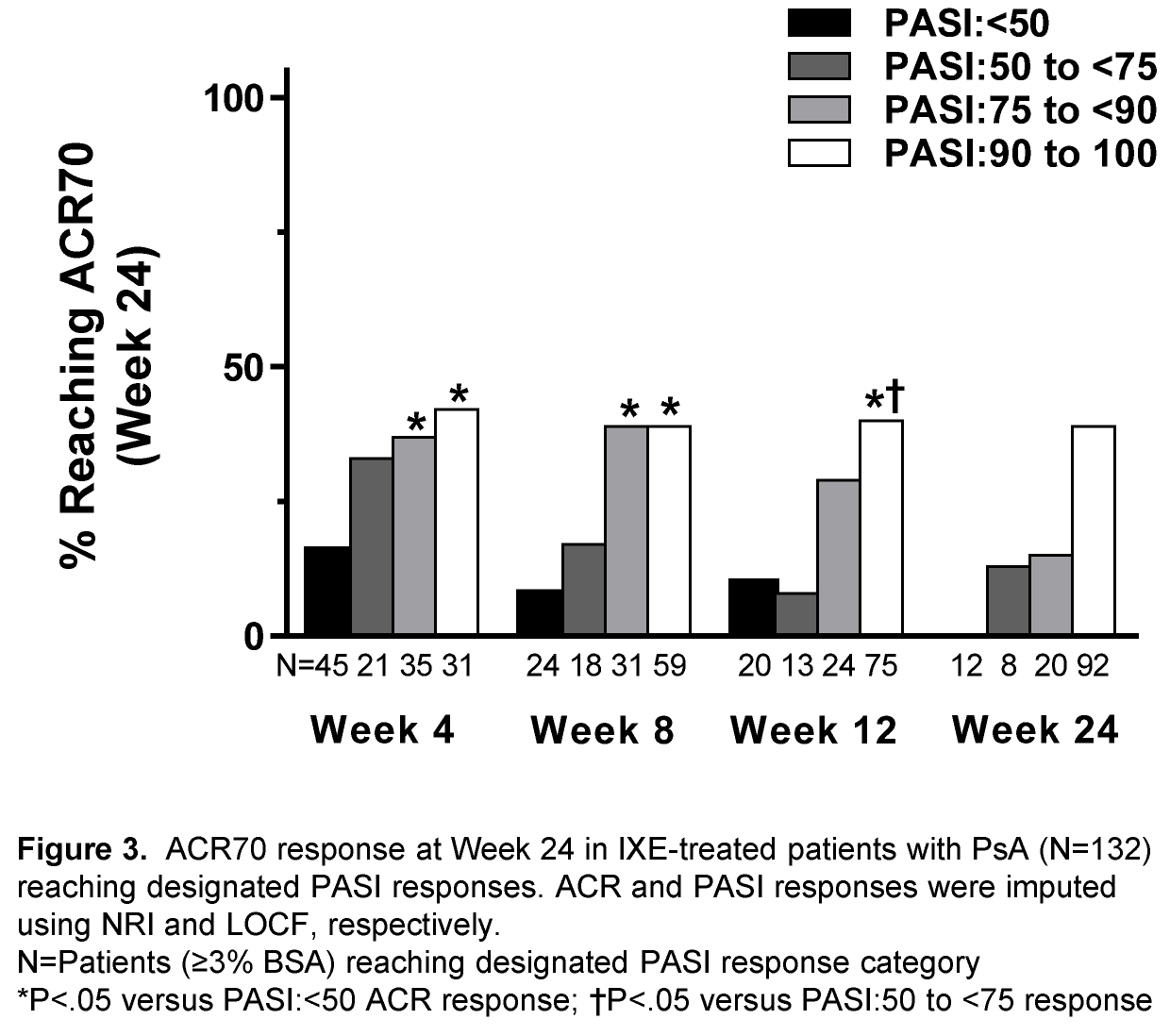
Results: Of the 417 PsA patients enrolled, 267 patients (69.5%) had ≥3% BSA at baseline. In IXE treated patients (Q2W and Q4W combined; N=132), patients with skin improvement of PASI≥50 at Wk 4 had greater ACR20/ACR50/ACR70 responses at Wk 24 than patients who had a PASI<50 response at Wk 4 (see Figures). Similar observations were made in patients with ≥10% BSA (data not shown). For IXE- and ADA-treated patients, greater PASI improvement by Wk 24 paralleled greater ACR responses at Wk 24 (See Figures; data not shown).
Conclusion: In PsA patients with moderate-to-severe Ps, greater improvement of psoriatic lesions at Wk 24 of biologic treatment was associated with greater reductions in PsA disease activity as assessed by ACR responses. In IXE treated patients, early PASI responses were predictive of ACR responses at Wk 24. 1. Mease et al. ACR/ARHP Annual Meeting 2015; [abstract 977]
To cite this abstract in AMA style:
Thaci D, Morita A, Birt J, Lin CY, Shuler CL, Gottlieb AB. Association of Early Skin Improvement with ACR Responses Among Biologic DMARD-Naive Psoriatic Arthritic Patients Treated with Ixekizumab [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/association-of-early-skin-improvement-with-acr-responses-among-biologic-dmard-naive-psoriatic-arthritic-patients-treated-with-ixekizumab/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/association-of-early-skin-improvement-with-acr-responses-among-biologic-dmard-naive-psoriatic-arthritic-patients-treated-with-ixekizumab/