Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: People with osteoarthritis (OA) commonly experience periods of increased pain and stiffness (“flares”), which can be distressing and disabling. Despite the prevalence of OA flares and their significant impact on quality of life, the mechanisms behind flares are still poorly understood. Whether COVID-19 vaccination triggers OA flares is unknown. In this study, we evaluated the association of COVID-19 vaccination with OA flares using a case-crossover design.
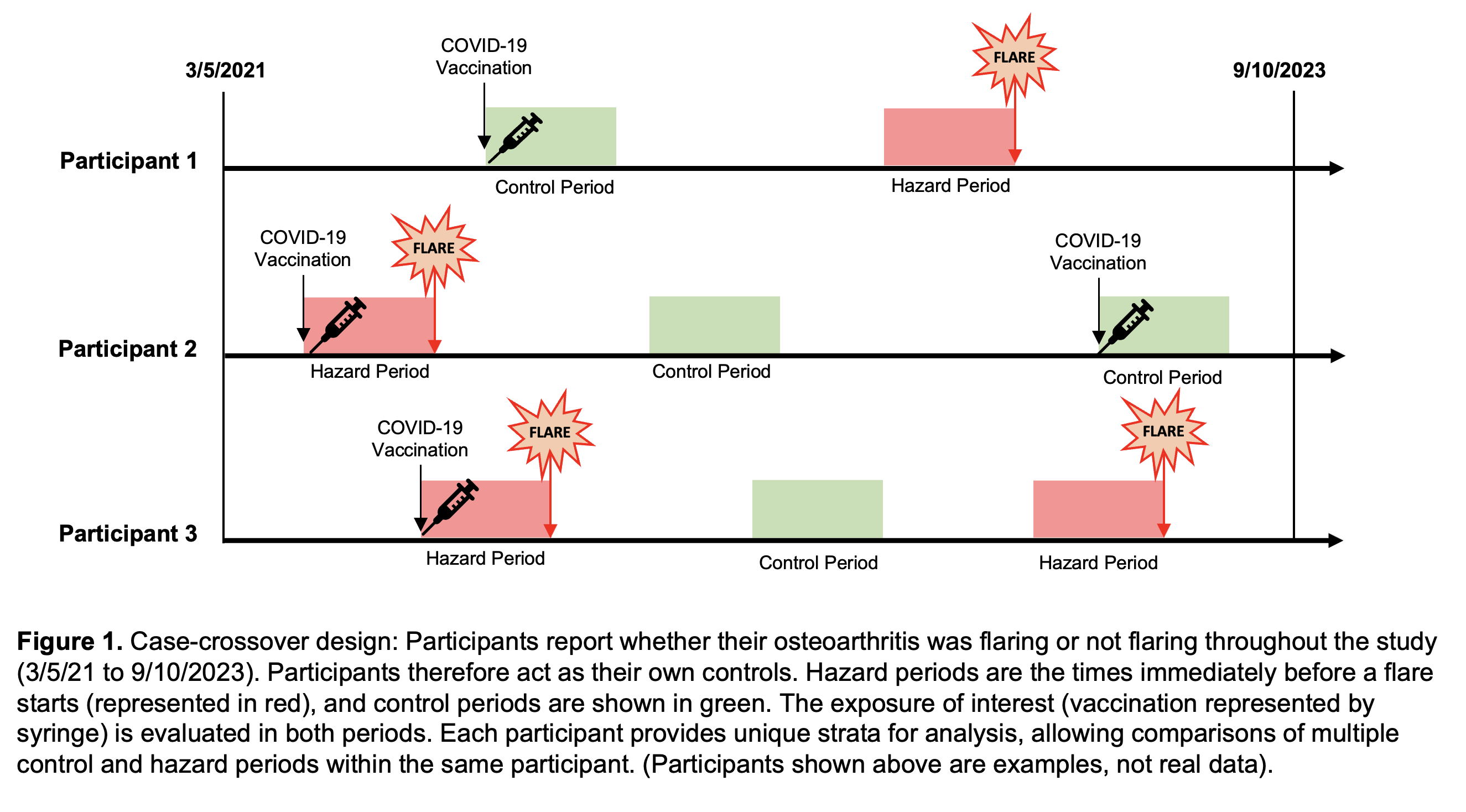
Methods: Adults ≥18 years enrolled in a COVID-19 Rheumatology Registry at a tertiary center were invited to participate. COVID-19 vaccine data were collected by self-report and cross-checked using electronic health records linked to the New York City Immunization Registry and Surescripts pharmacy network. OA diagnosis was determined using International Classification of Disease-10 algorithms; patients with algorithm-identified systemic rheumatic disease (SRD) were excluded. OA diagnosis and affected anatomic locations were chart-validated using physician notes and imaging reports. In the case-crossover design, participants reported flare (“hazard”) periods and non-flare (“control”) periods between 3/5/2021 and 9/10/2023 (Figure 1). Vaccine exposures in the 2-, 7-, and 14-day windows before flare periods were compared to vaccine exposures in 2-, 7-, and 14-day windows before non-flare periods, using a univariate conditional logistic regression model stratified by participant with a log-likelihood equivalent Cox proportional hazards model.
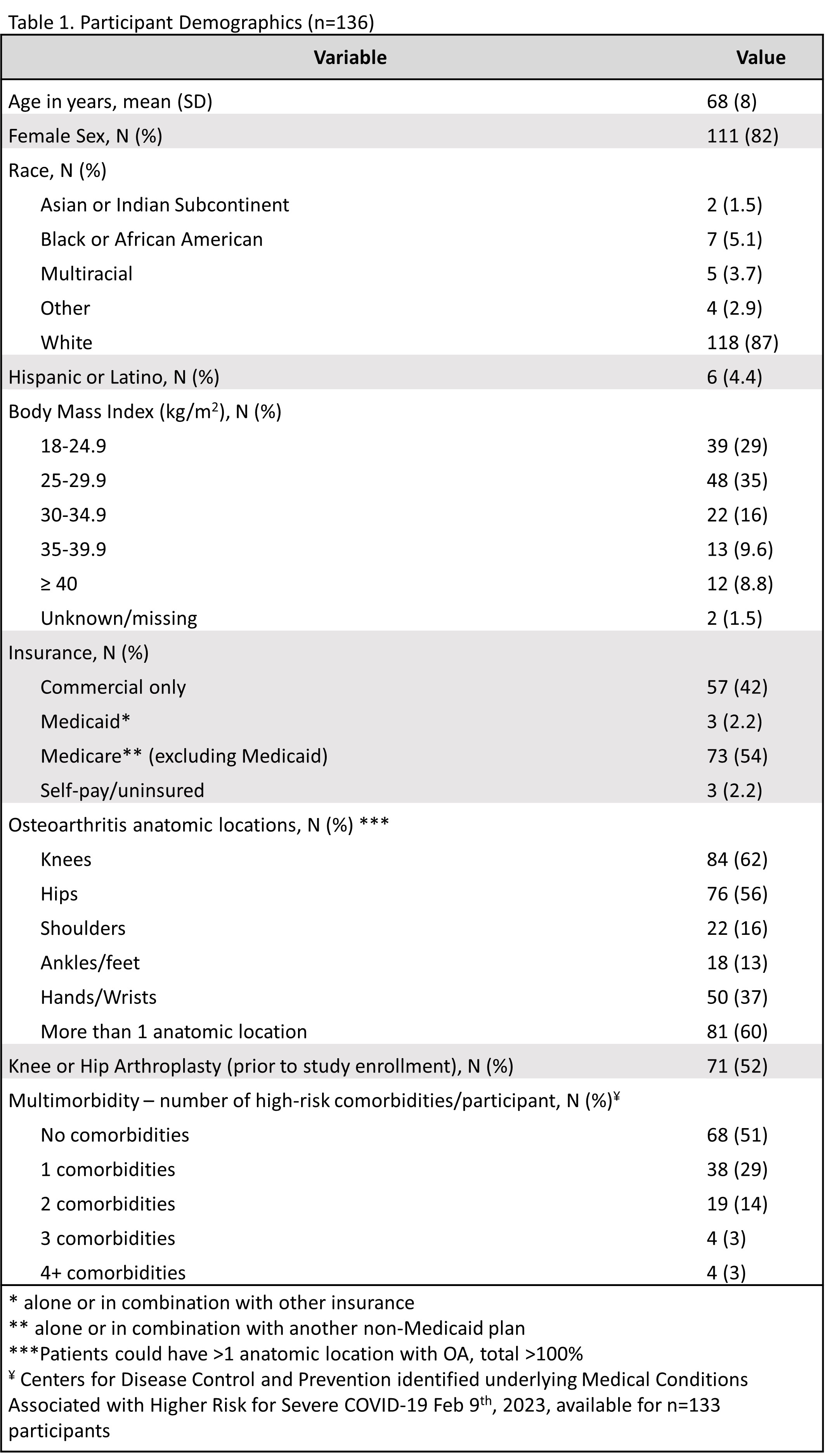
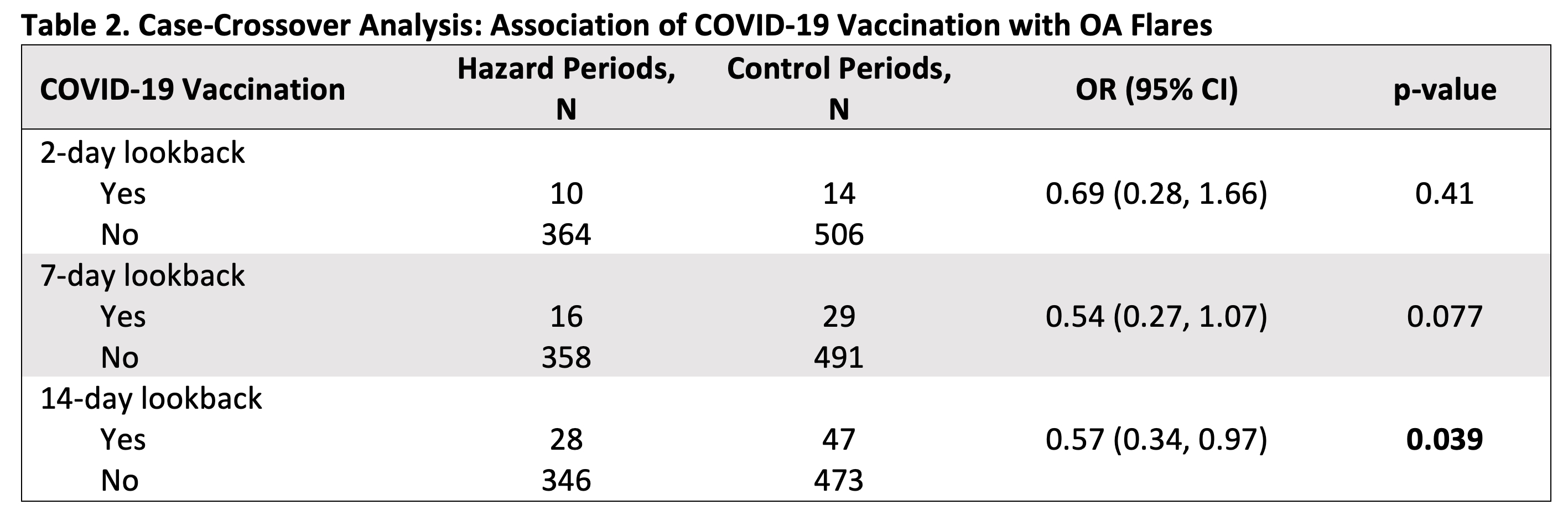
Results: 1797 (19%) registry participants opted into the flare study. After excluding for SRD, 279 had chart-validated OA, and 136 (49%) contributed at least one flare and one control period. Mean age was 68 years [SD ±8], and subjects were 82% female, 87% White (Table 1). OA distribution was 62% knee, 56% hip, 37% hand/wrist, 16% shoulder, and 13% ankle/foot; 60% of participants had more than one anatomic location affected, and 52% had a knee or hip arthroplasty. Of 525 COVID-19 vaccine doses recorded, 25% were 1st dose, 25% were 2nd, 20.5% were 3rd, and 29.5% were ≥ 4th. 52% were Pfizer-BioNTech, 46% were Moderna, and 2% were Johnson & Johnson, AstraZeneca, or missing. Participants reported 374 osteoarthritis flares and 520 control periods total. On a self-reported 3-tier severity scale, 30% flares were mild, 55% were moderate, and 14% were severe. OA flares were not associated with COVID-19 vaccination using lookback windows of 2 or 7 days (OR 0.69 [95% CI: 0.28, 1.66], OR 0.54 [95% CI: 0.27, 1.07], respectively). In the 14-day lookback window, proportionally fewer flares occurred after vaccination (OR 0.57 [95% CI: 0.34, 0.97], p=0.039) (Table 2). Subanalyses stratified on sex, age, knee or hand osteoarthritis, vaccine brand, and dose showed no positive association between COVID-19 vaccination and OA flares.
Conclusion: These findings provide reassurance that COVID-19 vaccination does not appear to trigger OA flares. The negative association between report of flare and vaccination in the preceding 14 days may suggest that OA patients are cautious and wait until periods of lower disease activity to schedule their vaccines.
To cite this abstract in AMA style:
Nong M, Lewis V C, Braverman G, Bykerk V, Hupert N, Barbhaiya M, Mandl L. Association of COVID-19 Vaccinations with Osteoarthritis Flares: A Case-Crossover Study [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/association-of-covid-19-vaccinations-with-osteoarthritis-flares-a-case-crossover-study/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/association-of-covid-19-vaccinations-with-osteoarthritis-flares-a-case-crossover-study/