Session Information
Session Type: Abstract Session
Session Time: 4:00PM-5:30PM
Background/Purpose: Fear of flare contributes to COVID-19 vaccine hesitancy in patients with systemic rheumatic diseases (SRD). Accurately ascertaining post-vaccine SRD flares and differentiating them from routine vaccine side effects is difficult. We evaluated the association of COVID-19 vaccination with SRD flares using a case-crossover design, which is ideal for studying outcomes triggered by transient within-person exposures with short windows of time-to-effect.
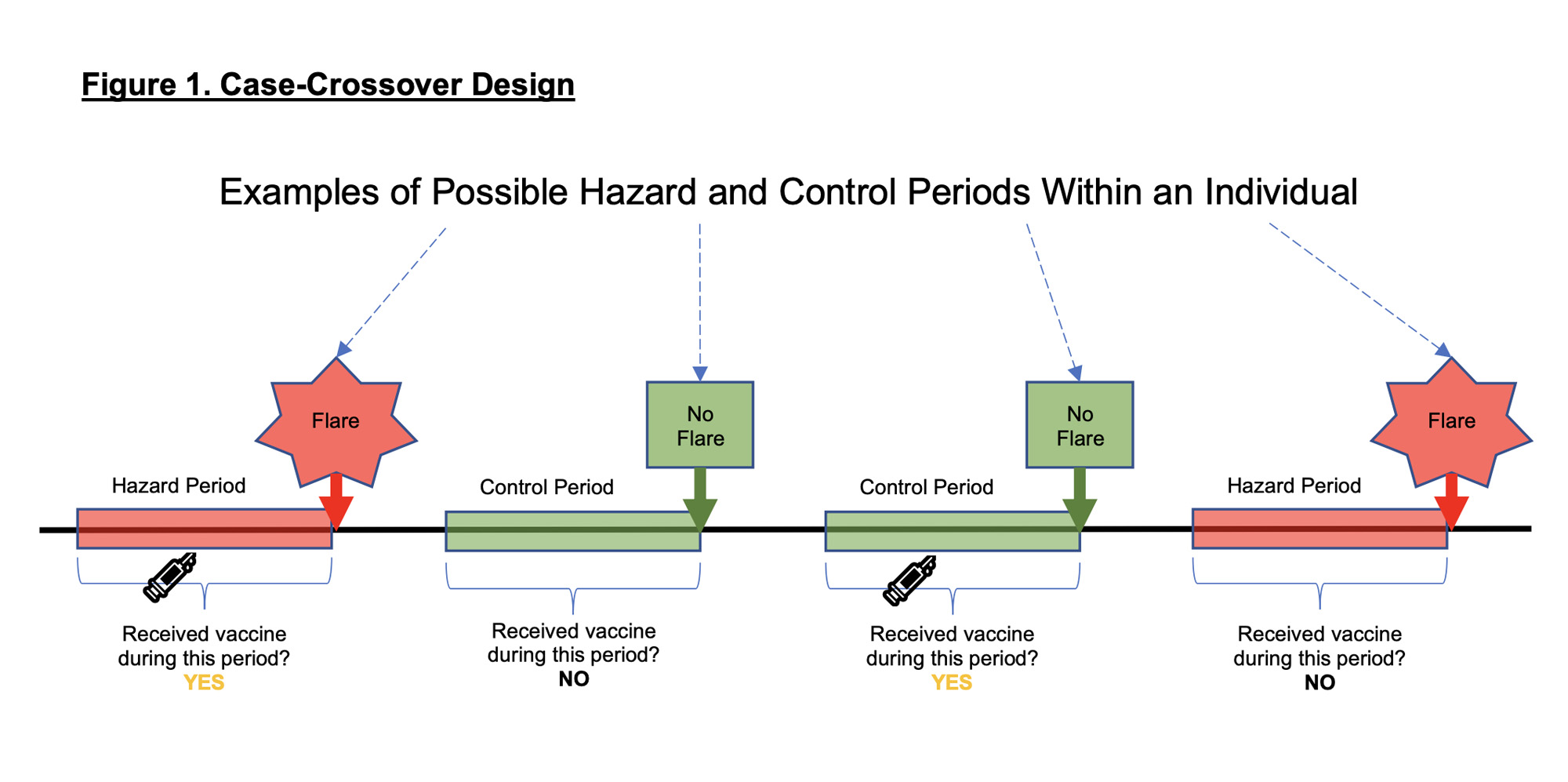
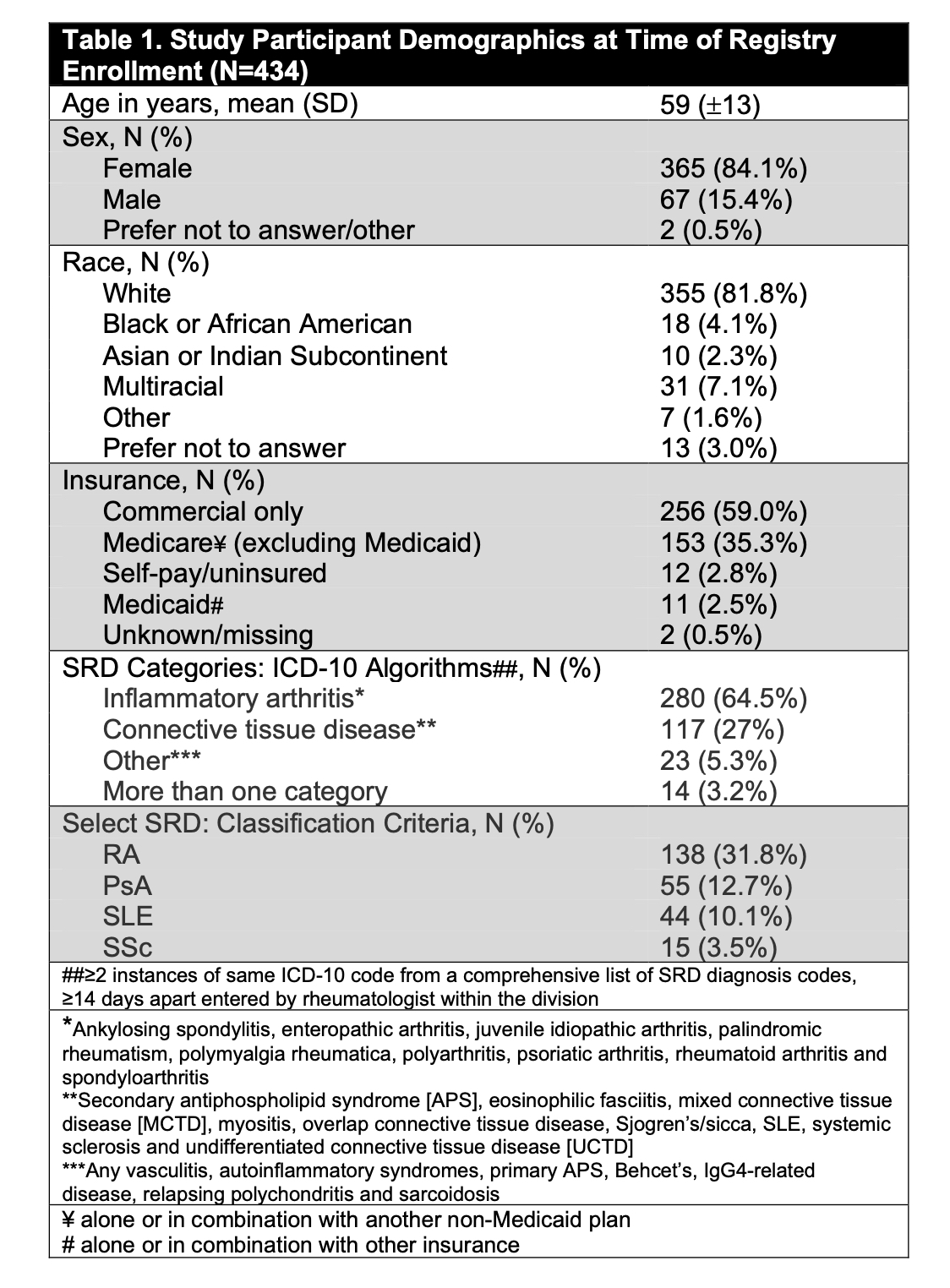
Methods: Adults ≥18 years enrolled in a COVID-19 Rheumatology Registry at a tertiary center were invited to participate in this study assessing SRD flares. COVID-19 vaccine dates and brands were obtained from self-report and electronic health records. International Classification of Disease-10 algorithms identified SRD diagnoses. Subjects with rheumatoid arthritis (RA), psoriatic arthritis (PsA), systemic lupus erythematosus (SLE) and scleroderma (SSc) had their diagnoses validated by classification criteria; 138 (31.8%), 55 (12.7%), 44 (10.1%) and 15 (3.5%) of 434 participants met criteria for RA, PsA, SLE and SSc, respectively. Participants self-reported both SRD flares and SRD quiescence. “Hazard periods” were defined as time before self-reported flares, and “control periods” as time before self-report of no flare. We used univariate conditional logistic regression, stratified by participant with a log likelihood equivalent Cox proportional hazards model, to evaluate for an association between flare and vaccination in the preceding 2, 7 and 14 days. (Figure 1). Subgroup analyses were performed.
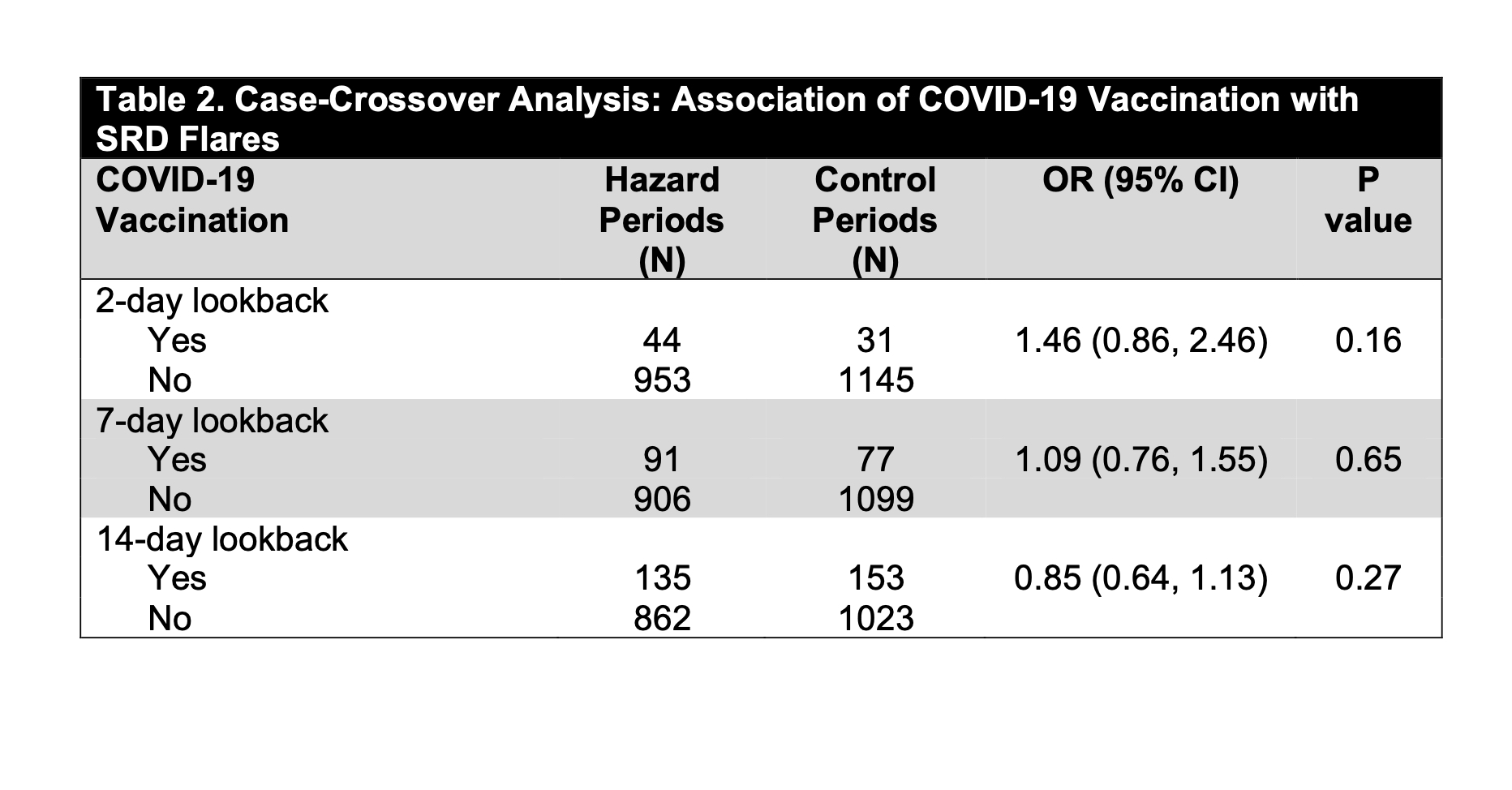
Results: Of 9361 Registry participants, 1797 opted into the SRD flare study and 434 (24.2%) subjects (mean age 59 years [±13], 84.1% female, 81.9% White) contributed both hazard and control periods between 3/5/21 and 9/6/22. Most had an inflammatory arthritis or a connective tissue disease (Table 1). 96.1% of subjects received at least 1 dose of a COVID-19 vaccine and 93.1% received at least 2 doses. Of the 1316 COVID-19 immunizations received during the study, 31.7% were 1st doses, 30.7% 2nd doses and the remainder ≥ 3rd doses; 58.5% were Pfizer-BioNTech, 39.5% were Moderna and 1.4% were Johnson & Johnson. 997 flares were reported. There was no association between COVID-19 vaccination and SRD flares using lookback windows of 2, 7 or 14 days (OR 1.46 [95% CI, 0.86-2.46], OR 1.09 [95% CI, 0.76-1.55], OR 0.85 [95% CI, 0.64-1.13] respectively; p=NS for all) (Table 3). Subanalyses stratified on sex, age, SRD subtype and vaccine brand similarly showed no association with flare. Information on vaccine side effects was available for 897/1316 (68.2%) doses; 468 (52.2%) and 491 (54.7%) of 897 doses were complicated by CDC-defined local and systemic vaccine side effects, respectively. There was no association between reporting a vaccine side effect and reporting an SRD flare (p=0.09). Information on whether reported flares were consistent with a subject’s “typical” flare was available for 813/997 (81.5%) flares, of which 664/813 (81.7%) were typical.
Conclusion: In this cohort of participants with SRD and high vaccine uptake, COVID-19 vaccination was not associated with SRD flares. These data are reassuring and can inform shared decision-making on COVID-19 immunization.
To cite this abstract in AMA style:
Braverman G, Barbhaiya M, Nong M, Bykerk V, Hupert N, Lewis V C, Mandl L. Association of COVID-19 Vaccinations with Flares of Systemic Rheumatic Disease: A Case-Crossover Study [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/association-of-covid-19-vaccinations-with-flares-of-systemic-rheumatic-disease-a-case-crossover-study/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/association-of-covid-19-vaccinations-with-flares-of-systemic-rheumatic-disease-a-case-crossover-study/