Session Information
Date: Monday, November 18, 2024
Title: Muscle Biology, Myositis & Myopathies – Basic & Clinical Science Poster III
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
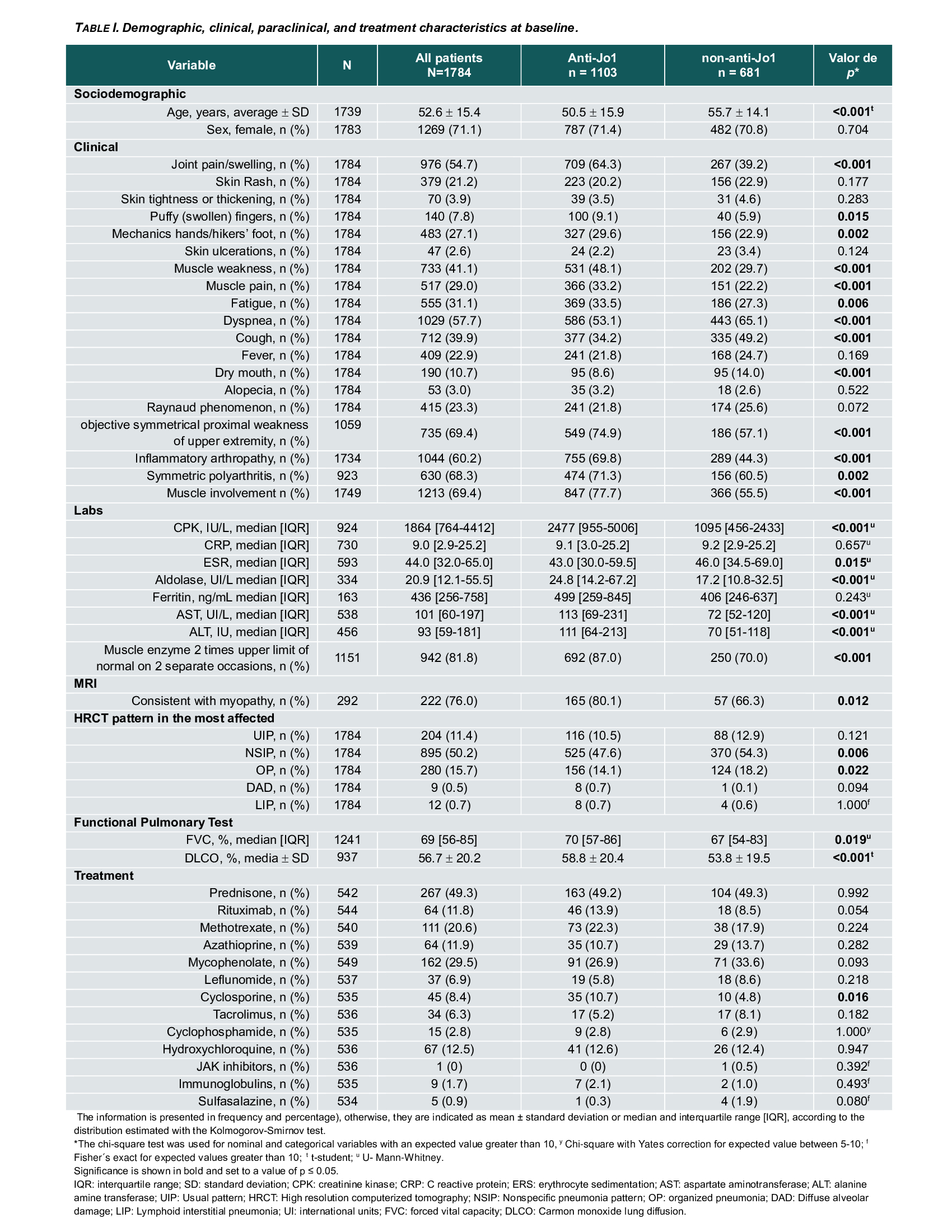
Background/Purpose: In anti-synthetase syndrome (ASSD), clinical presentations vary from isolated interstitial lung disease (ILD) to systemic multi-organ manifestations. Several studies emphasize the crucial role of the antibody subtype in determining the clinical features, survival rates, and High resolution computed tomography (HRCT) patterns of ILD between anti-Jo-1 and non-Jo-1 ASSD-ILD patients. The aim of the study was to compare the demographic, clinical, laboratory and radiographic profiles of Jo-1 antibody-positive versus non-Jo-1 antibody-positive ILD patients and the associations between antibody subtype on ILD evolution.
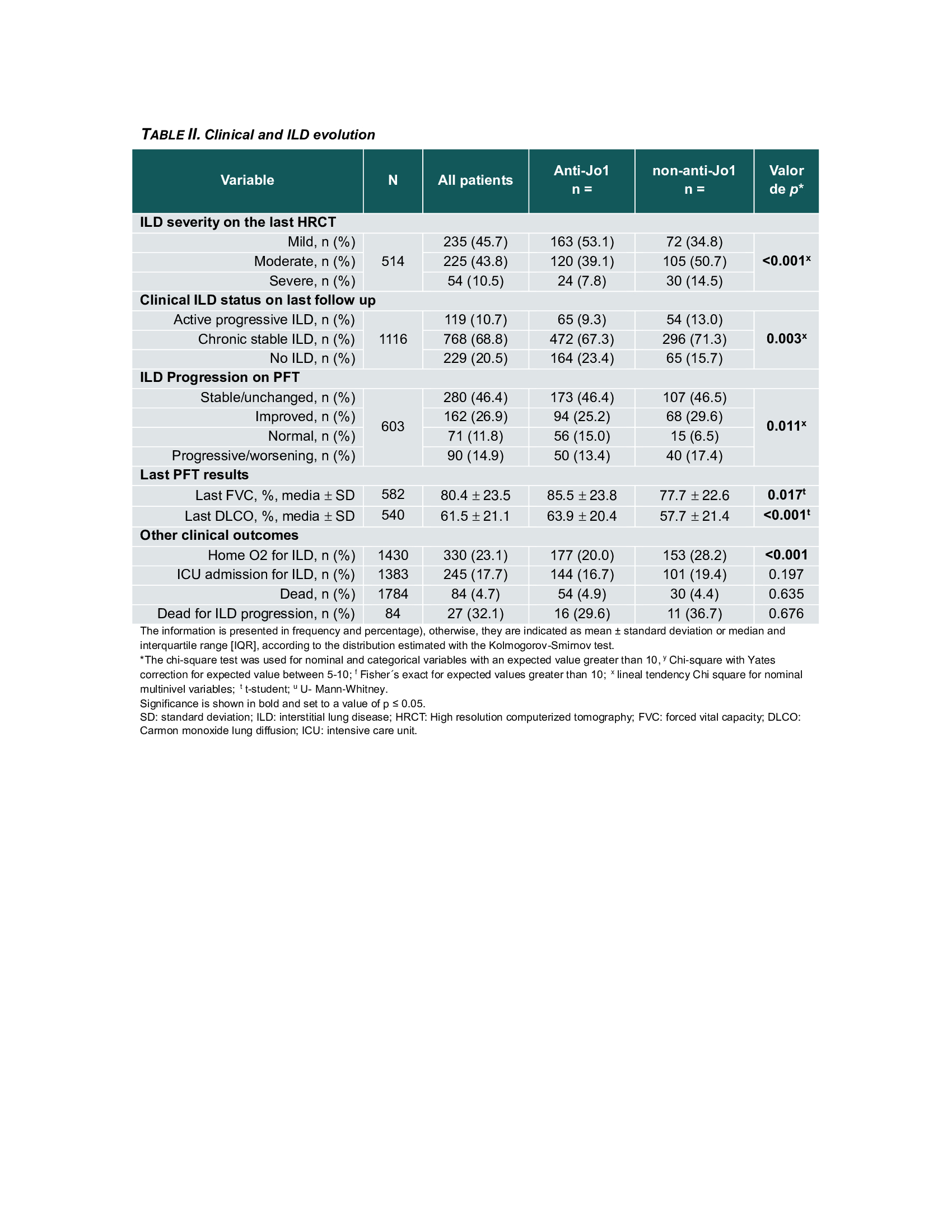
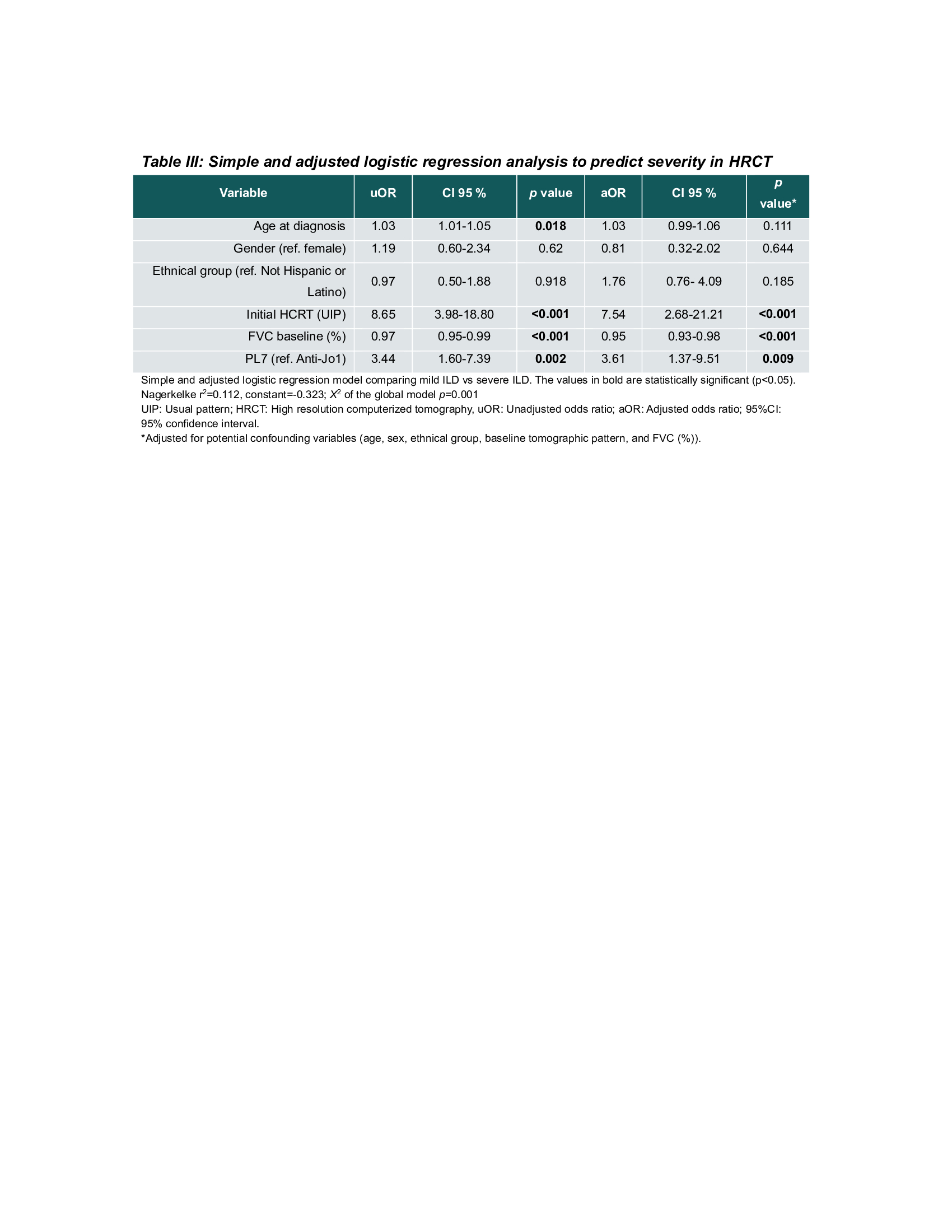
Methods: The Classification Criteria for Anti-Synthetase Syndrome (CLASS) project database was used as the primary data source, with data collected from 92 centers in 30 countries. Cases were classified by the treating physician and validated by the CLASS core team based on the clinical picture; local anti-ARS antibodies detected by any method were also considered. To compare characteristics between anti-Jo-1 and non-Jo-1 patients, the Chi-square test was used to analyze categorical variables, and the Student’s t-test or Mann-Whitney U-test was used for continuous variables, depending on their distribution. The severity of ILD involvement was assessed on the last HRCT evaluated by the treating physician in those patients with follow-up data. It was classified as mild, moderate and severe. A multivariate logistic regression model was performed to identify possible associations of ILD severity at radiographic follow-up with antibody subtype and adjusted for age, sex, ethnicity, baseline forced vital capacity (FVC), and HRCT pattern.
Results: A total of 1784 cases were included, 1103 Jo-1(62%) and 687 non- Jo-1(38%); mean age was lower in the Jo-1subgroup, 50.5 vs. 55.7 years. Anti-Jo-1 patients showed a higher frequency of mechanic’s hands and musculoskeletal manifestations. Non-Jo-1 patients had more frequent respiratory symptoms and lower lung function (DLCO and FVC; Table 1).A follow-up HRCT and pulmonary function test (PFTs) data was available in 514 (28.8%) and 603 (33.8%) patients, respectively. After 1 to 2 years from initial presentation, 45.7% of all patients were identified as having mild, 43.8% moderate, and 10.5% severe ILD. Severe ILD on HRCT was more frequent in non-Jo-1(14.5% vs. 7.8%, p< 0.001). There was also a higher frequency of ILD clinical progression (13.0 vs. 9.3, p=0.003) and ILD progression on PFTs (17.4% vs. 13.4%, p=0.01) in non-Jo-1 patients (Table 2). In the multivariate analysis, the variables associated with the severity of ILD on HRCT at follow-up were UIP pattern (aOR: 7.54; 95% CI: 2.68-21.21; p=< 0.001), baseline FVC (aOR: 0.95; 95% CI: 0.93-0.98; p=< 0.001) and PL-7 antibody positivity (aOR: 3.61; 95% CI: 1.37- 9.51; p=0.009) (Table 3).
Conclusion: Non-Jo-1 patients have a higher frequency of respiratory symptoms and more severe ILD than Jo-1 patients. In addition, PL-7 antibody positivity independently predicts the radiographic severity of ILD during follow-up.
To cite this abstract in AMA style:
Rivero Gallegos D, Bozan F, Bae S, Zanframundo G, Faghihi-Kashani S, Bauer Ventura I, Dourado E, sambataro G, Yoshida A, J Corte T, Bonella F, J Doyle T, fiorentino d, Gonzalez-Gay M, Hudson m, Kuwana M, Notarnicola A, Mammen A, McHugh N, Miller F, Montecucco C, Oddis C, Rojas-Serrano J, Schmidt J, Scire C, Selva-O’Callaghan A, Werth V, Aggarwal R, Cavagna L. Association of Anti-Synthetase Antibody Subtypes with Radiographic Progression of Interstitial Lung Disease in Anti-Synthetase Syndrome: An Analysis of the CLASS Project Database [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/association-of-anti-synthetase-antibody-subtypes-with-radiographic-progression-of-interstitial-lung-disease-in-anti-synthetase-syndrome-an-analysis-of-the-class-project-database/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/association-of-anti-synthetase-antibody-subtypes-with-radiographic-progression-of-interstitial-lung-disease-in-anti-synthetase-syndrome-an-analysis-of-the-class-project-database/