Session Information
Session Type: Poster Session D
Session Time: 1:00PM-3:00PM
Background/Purpose: Psoriatic arthritis (PsA) is a chronic inflammatory disease involving joint and enthesis disease. Ultrasound (US) has demonstrated its useful tool for the diagnosis of PsA and monitoring of inflammatory lesions in PsA. Our purpose is to conduct a study using US to evidence inflammatory changes in the joint and in the entheses in patients with active PsA (aPsA) who started Apremilast in clinical practice. Main objective was to obtain a 20% reduction in the US index (UIC) at 12 months.
Methods: Phase IV, multicenter, prospective and open-label clinical trial conducted in 9 centres from 2018 to 2021. All patients signed the informed consent. Approval by the ethical committee was obtained, code PSA‐PI‐006421. PsA patients (≥ 2 swollen joints), and ≥ 2 joint US synovitis and ≥ 1 US enthesitis at screening were recruited. 52 weeks follow up (baseline, and 1, 6, 9, 12 months). US (joint, tendon & enthesis), clinic (SJC, TJC, LEI, PGA, PtGA), and biological parameters (ESR & CRP) were registered at each visit. US scans were scored according to Ficjan et al (1). Also, an ungueal (nail plate (NP) and subungueal thickness (ST)) US evaluation of 2nd, 3rd and 5th finger was done. We performed a descriptive analysis. Qualitative variables as number and percentage, and quantitative variables as median and interquartile range (IQR) were described. Confidence intervals were considered at 95%. The one-sample t-test was performed to determine whether the reduction between [UIC] before and after the intervention was greater than the reference value of 20%.
A generalized linear mixed effect model was fitted with clinical parameters as the outcome and duration of follow-up as the main covariate. Changes in clinical parameters over time were evaluated by means of the time slope. To consider the individual variability in clinical parameters, the model was adjusted for a random intercept effect.
1. Ficjan A, Husic R, Gretler J, Lackner A, Graninger WB, Gutierrez M, et al. Ultrasound composite scores for the assessment of inflammatory and structural pathologies in Psoriatic Arthritis (PsASon-Score). Arthritis Res Ther. 2014;16(1):1–13.
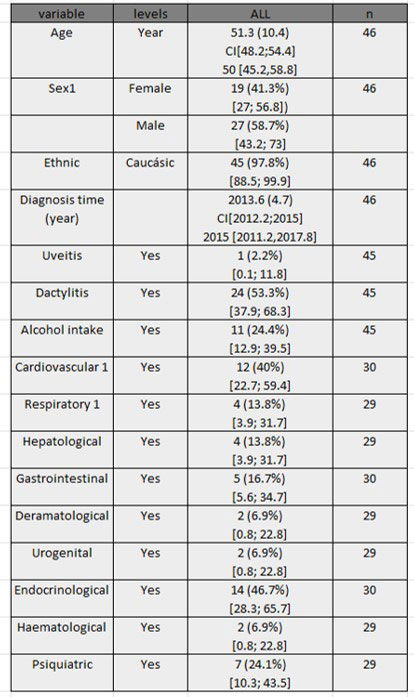
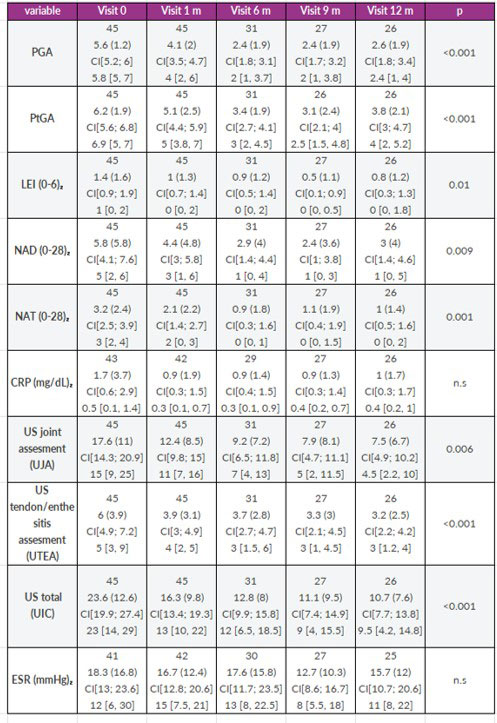
Results: 48 patients (3 excludes screening failure) screened and 46 included in follow up. 26 completed the 52 weeks study and were used for analysis. Baseline characteristics are in table 1. Main objective was achieved, and reduction was high until 40%. All, clinical and US variables (joint, tendon & enthesis), were significantly reduced after 12 months of follow-up (table 2). ESR and CRP did not change at follow up. Secondary objective, 50% of reduction was not achieved. A reduction in NP lesion, and slight relation between lesion, and high ST and ST with onychopathy was seen but didn’t achieve statistical significance (reduced sample size). 75 adverse events (AE) in 33 patients were register during study, only 1 was consider severe (SAE). Reasons for withdrawal were: 6 patients AE, 8 no efficacy, and 6 other reasons (loss of follow-up, withdrawal of consent).
Conclusion: Apremilast is a safe, well tolerated, and useful treatment in different patterns of PsA (joint, enthesis) demonstrated by an image ultrasonography score. Ultrasound can identify nail disease in PsA patients.
CI, coefficient interval
PGA, physician global assessment; PtGA, patient global assessment; LEI, Leeds enthesitis index; SJC, swollen joint count; TJC, tender joint count, CRP, c reactive protein; ESR; erythrosedimentation rate
To cite this abstract in AMA style:
de Agustin J, Añez G, reina d, Heredia S, Ramirez Garcia F, Cuervo A, Rodriguez J, Moragues C, Moya P, Moreno M, Arévalo M, Pujol M, Salvador G, Busquets N, Ponce a, Laiz Alonso A, Pascual Pastor M. Assessment of the Ultrasound and Clinical Response to Apremilast Using a Joint-periarticular-nail Ultrasound Index and Clinical Evaluation in Patients with Active Psoriatic Arthritis [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/assessment-of-the-ultrasound-and-clinical-response-to-apremilast-using-a-joint-periarticular-nail-ultrasound-index-and-clinical-evaluation-in-patients-with-active-psoriatic-arthritis/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/assessment-of-the-ultrasound-and-clinical-response-to-apremilast-using-a-joint-periarticular-nail-ultrasound-index-and-clinical-evaluation-in-patients-with-active-psoriatic-arthritis/