Session Information
Date: Sunday, October 26, 2025
Title: (0233–0279) Miscellaneous Rheumatic & Inflammatory Diseases Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: IgG4-related disease (IgG4-RD) is a progressive, systemic, fibroinflammatory disease characterized by unpredictable and recurring flares, leading to organ damage and decreased quality of life. Inebilizumab is a humanized, glycoengineered, CD19-directed, monoclonal antibody that depletes CD19+ B cells, including plasmablasts and some plasma cells, effectively in a targeted manner. MITIGATE (NCT04540497) is an international, randomized, blinded, placebo-controlled Phase 3 trial that evaluates the safety and efficacy of inebilizumab as treatment for IgG4-RD. The aim is to evaluate the safety and efficacy of inebilizumab across various baseline disease characteristic subgroups of participants enrolled in the study.
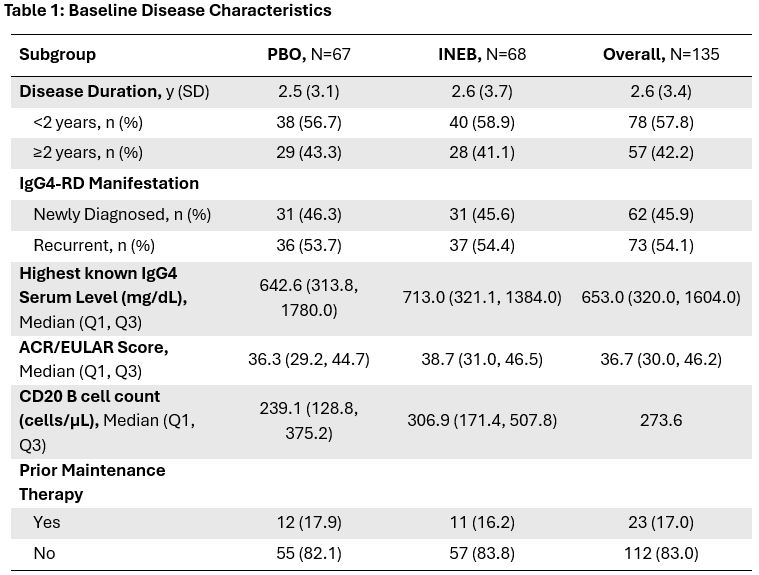
Methods: Adult participants who met the ACR/EULAR Classification Criteria for IgG4-RD with a score ≥20 were enrolled in the study. Eligible participants had a history of multiorgan IgG4-RD and had experienced an IgG4-RD flare that required glucocorticoid treatment during the screening period. Additional eligibility criteria and study design have been previously reported (Stone et al, 2024). The primary endpoint (time to first adjudication committee (AC)-determined and investigator-treated IgG4-RD flare during the RCP) and all key secondary endpoints were met in the overall study population. This post hoc subgroup analysis evaluated safety and efficacy of inebilizumab across baseline characteristics: disease duration, IgG4 serum level, ACR/EULAR score, CD20 B-cell count, and prior maintenance therapy.
Results: 135 participants were randomized and received at least one dose of inebilizumab (n=68) or placebo (n=67). Baseline disease characteristics were generally well balanced between the 2 treatment groups for subgroups (Table 1). Inebilizumab treatment substantially reduced the risk of flare relative to placebo in all subgroups (Table 2), with each subgroup exhibiting a hazard ratio similar to the overall study population. Annualized flare rates were reduced by inebilizumab relative to placebo in all subgroups. Proportions of participants achieving flare-free, treatment-free complete remission or flare-free, corticosteroid-free complete remission at Week 52 were increased by inebilizumab in all subgroups with sufficient representation to permit analysis (Table 3). No subgroup exhibited safety findings that were substantially different from those observed in the overall population.
Conclusion: The MITIGATE trial demonstrates the consistent benefit and safety of inebilizumab across all baseline disease characteristic subgroups analyzed. Inebilizumab represents a viable treatment option for all patients, regardless of their IgG4-RD baseline disease characteristics.
To cite this abstract in AMA style:
Tanaka Y, Culver E, Khosroshahi A, Zhang W, Okazaki K, Lohr M, schleinitz n, Dong X, rosen m, Cheng S, Cimbora D, Stone J. Assessment of Baseline IgG4-RD Disease Characteristics and Impact Upon Safety and Efficacy of Inebilizumab: Results from the MITIGATE Study [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/assessment-of-baseline-igg4-rd-disease-characteristics-and-impact-upon-safety-and-efficacy-of-inebilizumab-results-from-the-mitigate-study/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/assessment-of-baseline-igg4-rd-disease-characteristics-and-impact-upon-safety-and-efficacy-of-inebilizumab-results-from-the-mitigate-study/

.jpg)
.jpg)