Session Information
Session Type: Poster Session A
Session Time: 6:00PM-7:00PM
Background/Purpose: Since 2010, the Childhood Arthritis and Rheumatology Research Alliance (CARRA) has developed 12 consensus treatment plans (CTP) with the aim of reducing treatment variability and promoting the conduct of comparative effectiveness research (CER) utilizing the CARRA registry. In November 2021 CARRA created a multi-stakeholder CTP Task Force (TF) charged with assessing the existing CARRA CTP program and making recommendations for improvement and expansion. Here, we report the initial work on systematic assessment of barriers and facilitators to reliable and widespread use of the CARRA CTPs.
Methods: The CTP TF gathered data using surveys, qualitative interviews, brainstorming and feedback sessions. Electronic surveys were developed, beta tested and administered to providers (PR), research coordinators (RC), and caregivers. A clinical implementation science expert with qualitative research experience interviewed a group of PRs and RCs of diverse geography, CARRA site size and experience to further assess barriers and facilitators of CTP use. The interview guides adhered to implementation frameworks and organizational readiness. The team evaluated CARRA Registry data relevant to CTPs for participants with juvenile idiopathic arthritis (JIA), lupus and juvenile dermatomyositis (JDM). The CARRA Steering Committee provided feedback via structured, small-group discussions.
Results: The initial provider survey (n=154) showed that awareness about most CTPs was high; the vast majority utilized CTPs in clinical practice but strict adherence to CTPs was low. A primary barrier to use was inaccessibility of CTPs at point of care. A second provider survey (n=119) showed that awareness of newer CTPs was lower and affirmed that CTPs were frequently referenced but adhered to with variable fidelity. Survey of research coordinators (n=43) identified high awareness of CTPs but variation in documentation of CTP use in the Registry. Most caregivers were not aware of CTPs but were interested to learn more and support CTP use. Thirteen interviews were conducted (7 PR, 6 RC) and analyzed. Facilitators and barriers to implementation of CTPs, alignment with implementation framework constructs, and suggestions for future CARRA efforts are outlined in Table 1 and Table 2. CARRA Registry data analysis showed that opportunities exist to improve patient-reported and laboratory data collection. Data quality issues were common regardless of disease or CTP assessed. Site declaration of CTP use was inconsistent and often did not match the treatment received, though overall treatment patterns did not substantially differ between patients with or without a declared CTP. Collection of physician-generated outcomes data is excellent and on par with externally funded CER studies.
Conclusion: CTPs are valued and utilized by the CARRA community as clinical and research tools. Barriers exist to widespread CTP use, particularly accessibility at point of care. Improving data collection in the Registry may provide additional insights. The CTP TF developed a list of recommendations for a multi-year optimization process as outlined in Table 3.
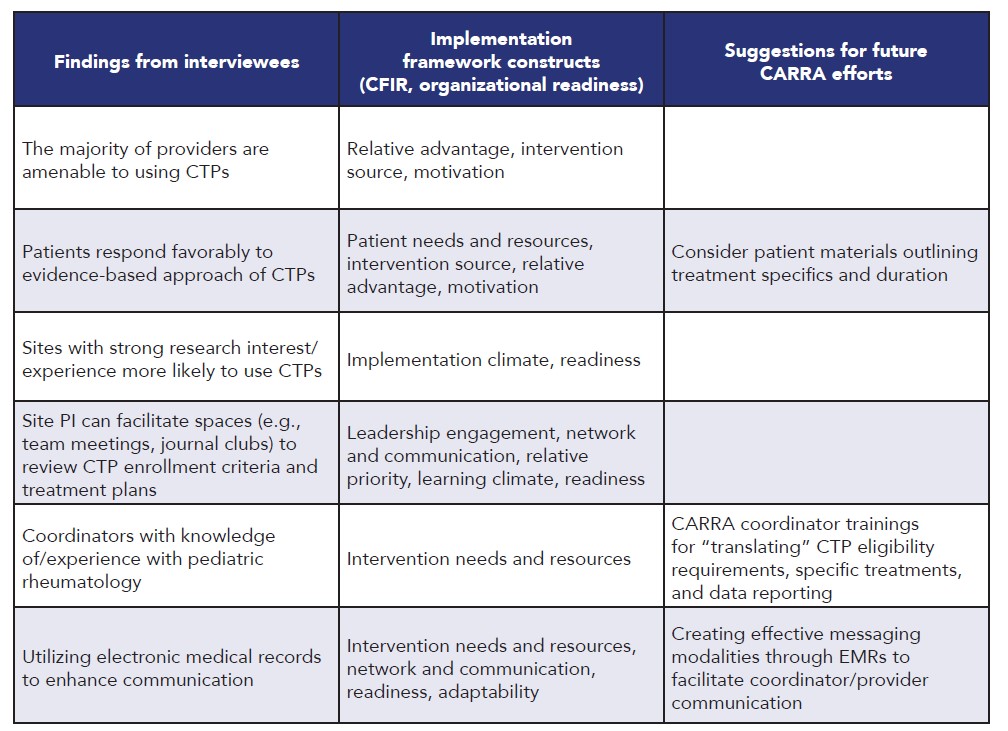 Table 1. Facilitators to Implementing CTPs
Table 1. Facilitators to Implementing CTPs
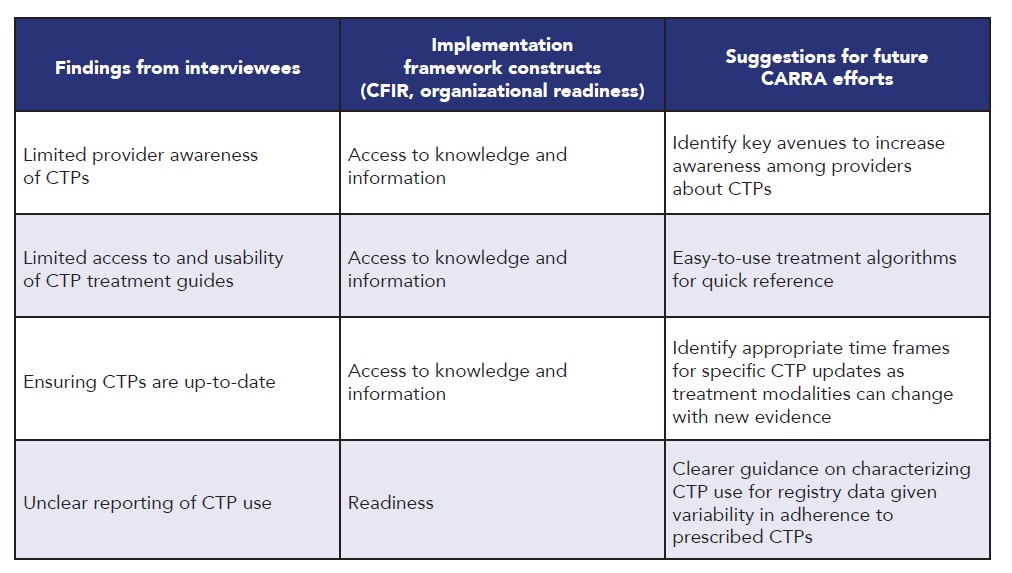 Table 2. Barriers to Implementing CTPs
Table 2. Barriers to Implementing CTPs
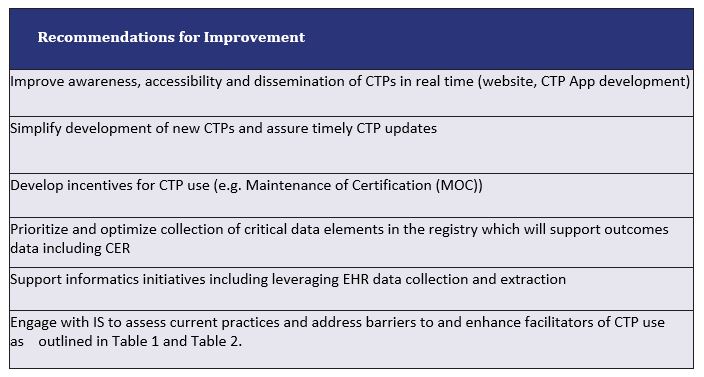 Table 3. The CTP TF Recommendations for Improvement
Table 3. The CTP TF Recommendations for Improvement
To cite this abstract in AMA style:
Yildirim-Toruner C, Glaser D, Beukelman T, Ardoin S, Hashmi A, Pooni R, Fernandez M, Del Gaizo V, Hanrahan L, Riordan M, Tarvin S, Investigators C. Assessment of Barriers and Facilitators in Implementation of the Childhood Arthritis and Rheumatology Research Alliance Consensus Treatment Plans [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 4). https://acrabstracts.org/abstract/assessment-of-barriers-and-facilitators-in-implementation-of-the-childhood-arthritis-and-rheumatology-research-alliance-consensus-treatment-plans/. Accessed .« Back to 2023 Pediatric Rheumatology Symposium
ACR Meeting Abstracts - https://acrabstracts.org/abstract/assessment-of-barriers-and-facilitators-in-implementation-of-the-childhood-arthritis-and-rheumatology-research-alliance-consensus-treatment-plans/
