Session Information
Date: Sunday, November 17, 2024
Title: SpA Including PsA – Diagnosis, Manifestations, & Outcomes Poster II
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Trials for patients with juvenile spondyloarthritis (JSpA) and axial disease are scarce. We assessed the responsiveness of measures for use in trials of patients with axial JSpA.
Methods: This was a 4-center, prospective, observational study of tumor necrosis factor inhibitor (TNFi)-naïve patients with JSpA and axial arthritis starting TNFi therapy. Participants were ages 8 to 18 years and met the International League of Associations for Rheumatology (ILAR) juvenile idiopathic arthritis criteria for enthesitis-related arthritis (ERA) or psoriatic arthritis (PsA) OR European Spondyloarthritis Study Group criteria for SpA. All subjects completed study visits (questionnaires and magnetic resonance imaging [MRI]) at baseline and 12 weeks after TNFi initiation. Subjects with continued subchondral sacroiliac joint (SIJ) inflammation on MRI at 12 weeks, as determined by 1 rater’s global assessment, completed an additional survey and MRI at 24 weeks. At study end, all MRI scans underwent central review by 3 raters; inflammation was scored using the Spondyloarthritis Research Consortium of Canada (SPARCC) SIJ inflammation score (SIS). Change scores and standardized mean difference (SMD) for all measures were calculated. Subjects were considered clinical responders if they reported meaningful improvement from the index visit.
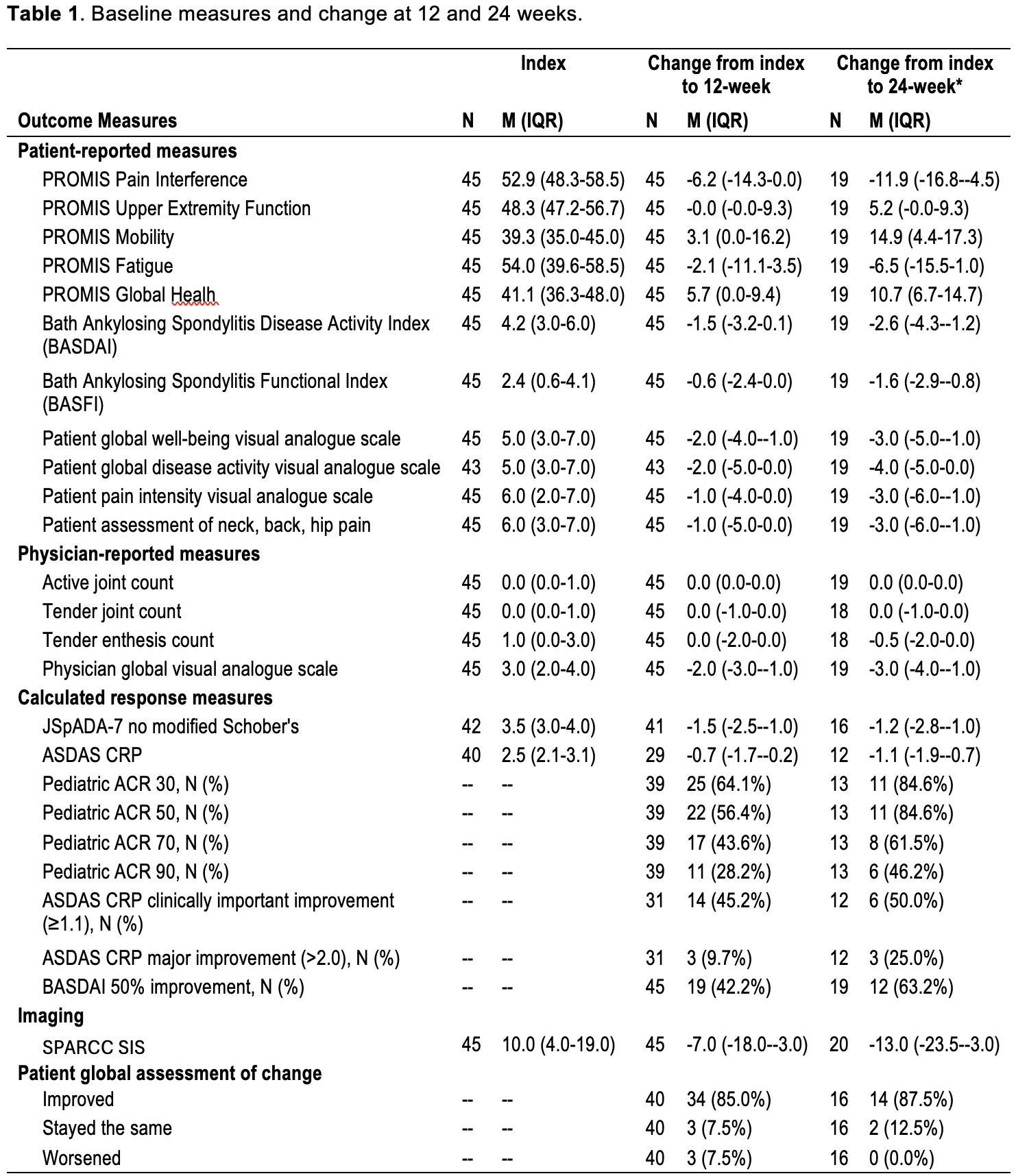
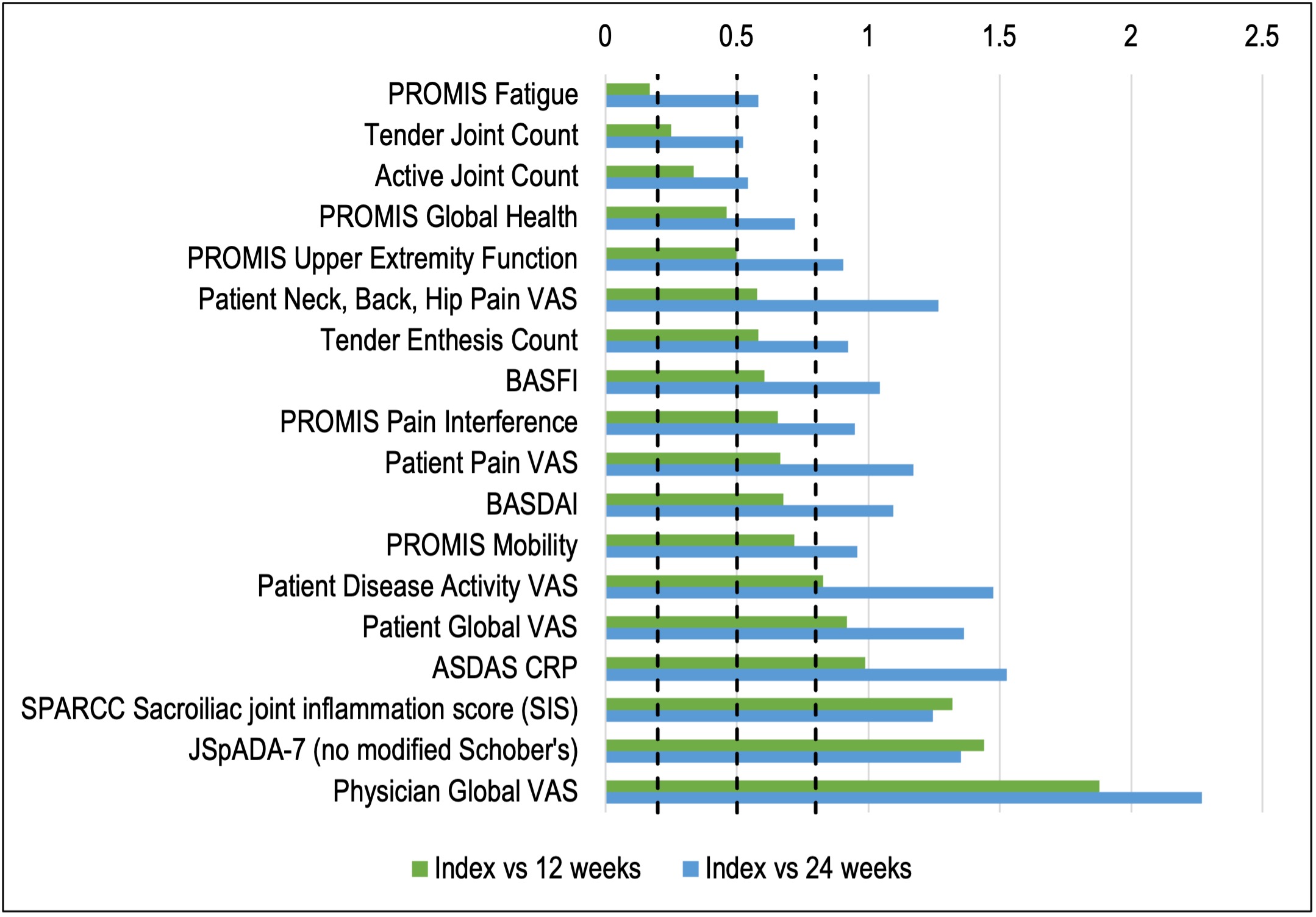
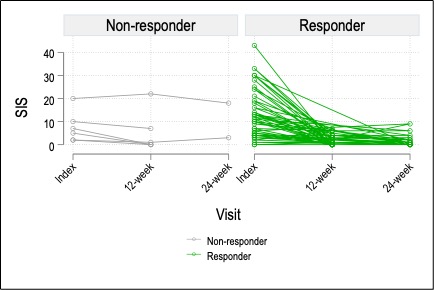
Results: 110 visits among 45 unique subjects were included. The cohort was 67% male, 76% white, and had a median age of 14.6 years (IQR 13.1-16.8). 44% were HLA-B27+ and 10% had a family history of SpA. 38% had peripheral arthritis and 66% had enthesitis. Table 1 shows change scores and proportions achieving response outcomes. At the 12-week visit, 80% of youth self-reported a global improvement of moderate or large importance to TNFi therapy and 64.1%/56.4%/43.6%/28.2% met criteria for the ACR 30/50/70/90, respectively. MRI SIS improved from index visit to week 12 in 89% of patients. 22/45 (48.9%) and 12/20 (60.0%) had continued inflammation on MRI at 12 and 24 weeks, respectively. Of those with continued MRI inflammation at 12 weeks, the median SIS and SIS change from index visit were 3 and -7, respectively, and 77% reported clinical improvement of at least moderate importance. SMDs of each measure are shown in Figure 1. Measures with the largest SMD between index and 12-week visit were physician global VAS (1.9), JSpADA-7 index (1.4), and MRI SIS (1.3). Change in the SIS is shown in Figure 2 by clinical responder status; most of the improvement (84% of total baseline SIS across all subjects) occurred between the index visit and week 12. Of the 6 clinical non-responders, only 2 had a worsening of the SIS.
Conclusion: 80% of youth with SpA and sacroiliitis had a clinical response of moderate or large importance to TNFi therapy by 12 weeks. Half of the subjects had continued MRI inflammation at 12 weeks, 77% of which reported clinical improvement of at least moderate importance. Standard pediatric clinical trial calculated response metrics may underestimate clinical response in children with axial disease, underscoring the need for a more responsive set of criteria for this population.
*At the 24-week visit, 20 subjects completed the MRI, 19 subjects completed the study questionnaires, and 19 subjects underwent physical examination during a routine clinic appointment.
To cite this abstract in AMA style:
Brandon T, Xiao R, Muir C, Stoll M, Lovell D, Oberle E, Chauvin N, Francavilla M, Maksymowych W, Weiss P. Assessing Responsiveness of Outcome Measures for Children with Axial Juvenile Spondyloarthritis (JSpA) [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/assessing-responsiveness-of-outcome-measures-for-children-with-axial-juvenile-spondyloarthritis-jspa/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/assessing-responsiveness-of-outcome-measures-for-children-with-axial-juvenile-spondyloarthritis-jspa/