Session Information
Date: Sunday, October 26, 2025
Title: (0554–0592) Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: The recently updated Assessment of SpondyloArthritis international Society (ASAS) core outcome set (COS)includes an agreed minimum set of instruments that should be used in all axial spondyloarthritis (axSpA) trials.However, recommendations on how to report outcomes of the 16 COS instruments is lacking.Aim of the project is to develop expert endorsed recommendations on how outcomes of COS instruments should be reported.
Methods: A steering group (SG) (FvG, VNC, DvH) convened a workgroup (WG) consisting of thirteen ASAS membersincluding rheumatologists, methodologists, epidemiologists, and two Young ASAS (Y-ASAS) members (all otherco-authors). Recommendations on reporting instrument outcomes were developed in three steps: the SGformulated a proposal on how outcomes related to the instruments should be presented. ii) the proposal waspresented, discussed and modified in a WG meeting. iii) the WG proposal was discussed and voted on by ASASmembers.
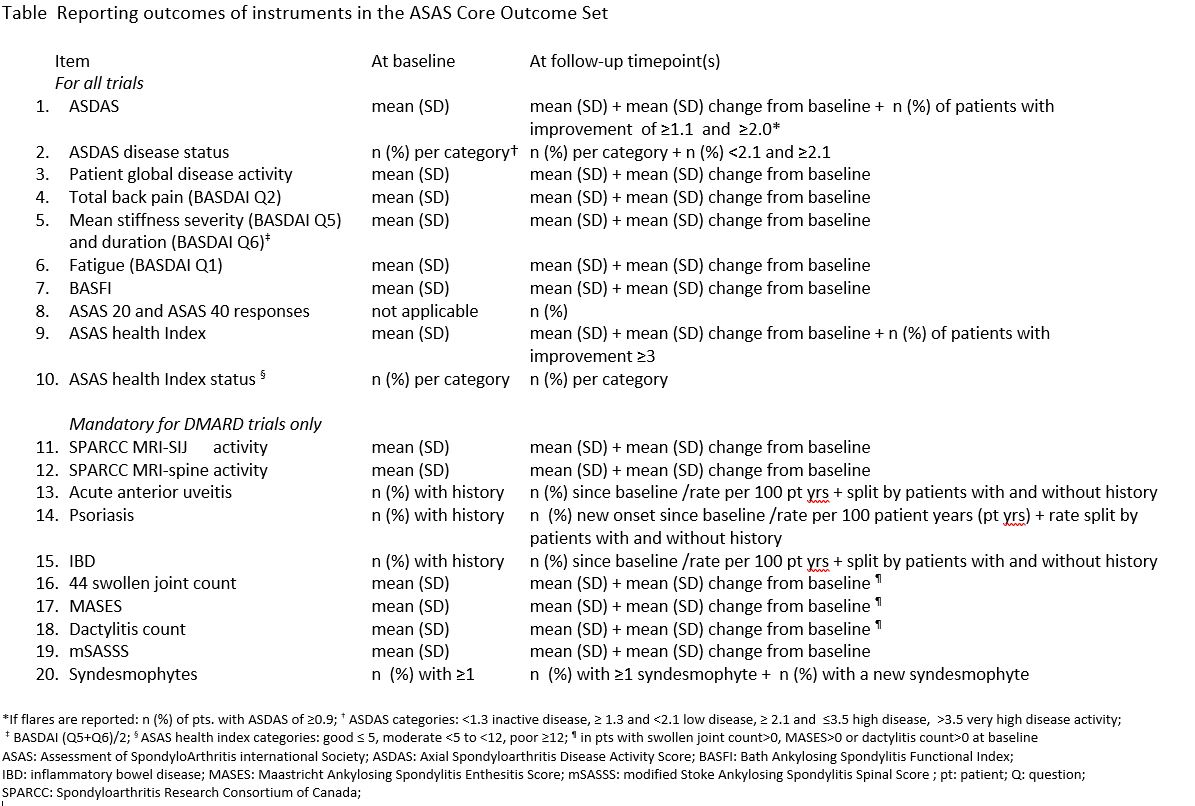
Results: Reporting 20 trial outcomes at baseline and follow-up timepoints are recommended: 10 for all clinical trials and10 for DMARD trials only (Table). The most common format is to report the mean plus the standard deviation(SD) at all timepoints while adding the mean change (SD) from baseline for each timepoint. For ASDAS it isrecommended to also report the number and percentage of patient with decrease of ≥1.1 and ≥2.0 units, anincrease of 0.9 for flares, and ASDAS status at all timepoints (inactive (< 1.3), low (≥1.3 and < 2.1), high (≥2.1 and≤3.5), and very high ( >3.5) plus the number and percentage of patients with scores < 2.1 and ≥2.1). For ASAShealth index it is recommended to also report the number and percentage of patients with decrease in thescore of ≥3 and status (good (≤ 5), moderate (< 5 to < 12), and poor (≥12)) at all timepoints.It is recommended for psoriasis, uveitis, and IBD to be reported as the number and percentage of patients withflare or (re)occurrence since baseline plus the rate per 100 patient-years while also splitting the results bypatients with and without a history of that particular extra-musculoskeletal manifestations. For spinalsyndesmophytes it is recommended to report the number and percentage of patients with at least one atbaseline and outcomes plus the number and percentage of patients with a newly developed syndesmophytewhich, if not scored separately, can be derived from the mSASSS scoring with a vertebral unit over timedeveloping an mSASSS score of 2 or 3 from a score less than 2 counting as the development of asyndesmophyte. At the 2024 annual ASAS workshop recommendations were voted in by 81 full ASAS memberswith 85% in favour, 9% against, and 6% abstaining.
Conclusion: These ASAS endorsed recommendations provide a consensual approach to reporting outcomes of axSpAclinical trials. Adherence to these recommendations will standardize presentation of results and therebyprovides better insight into trial outcomes and facilitate the comparison of outcomes across studies.
To cite this abstract in AMA style:
van Gaalen F, Navarro-Compan V, Baraliakos X, Van den Bosch F, Gensler L, Hmamouchi I, Landewé R, Machado P, Marzo-Ortega H, Rios Rodriguez V, Poddubnyy D, Ramiro S, Van Der Heijde D. ASAS recommendations on reporting outcomes of core outcome set instruments in axial spondyloarthritis clinical trials [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/asas-recommendations-on-reporting-outcomes-of-core-outcome-set-instruments-in-axial-spondyloarthritis-clinical-trials/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/asas-recommendations-on-reporting-outcomes-of-core-outcome-set-instruments-in-axial-spondyloarthritis-clinical-trials/