Session Information
Date: Tuesday, November 14, 2023
Title: (1913–1944) Miscellaneous Rheumatic & Inflammatory Diseases Poster III
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Immune checkpoint inhibitors (ICI) have changed the landscape of cancer treatment dramatically, but because of their mechanism of action they are also known to cause immune-related adverse events, including chronic inflammatory arthritis (ICI-IA) and osteoarthritis flares. Additionally, the safety of joint arthroplasty in such patients has not yet been described. We undertook this study to describe the histology and outcomes of hip and knee arthroplasty (THA and TKA) at our high-volume orthopedic hospital among patients who received ICI within one year of surgery.
Methods: We identified all ICI-treated patients who underwent joint arthroplasty at Hospital for Special Surgery (HSS) from 1/1/2018 to 12/31/2022, excluding patients whose last dose of ICI was >365 days prior to surgery. Clinical information was extracted retrospectively from the electronic medical record. The study was approved by the HSS IRB.
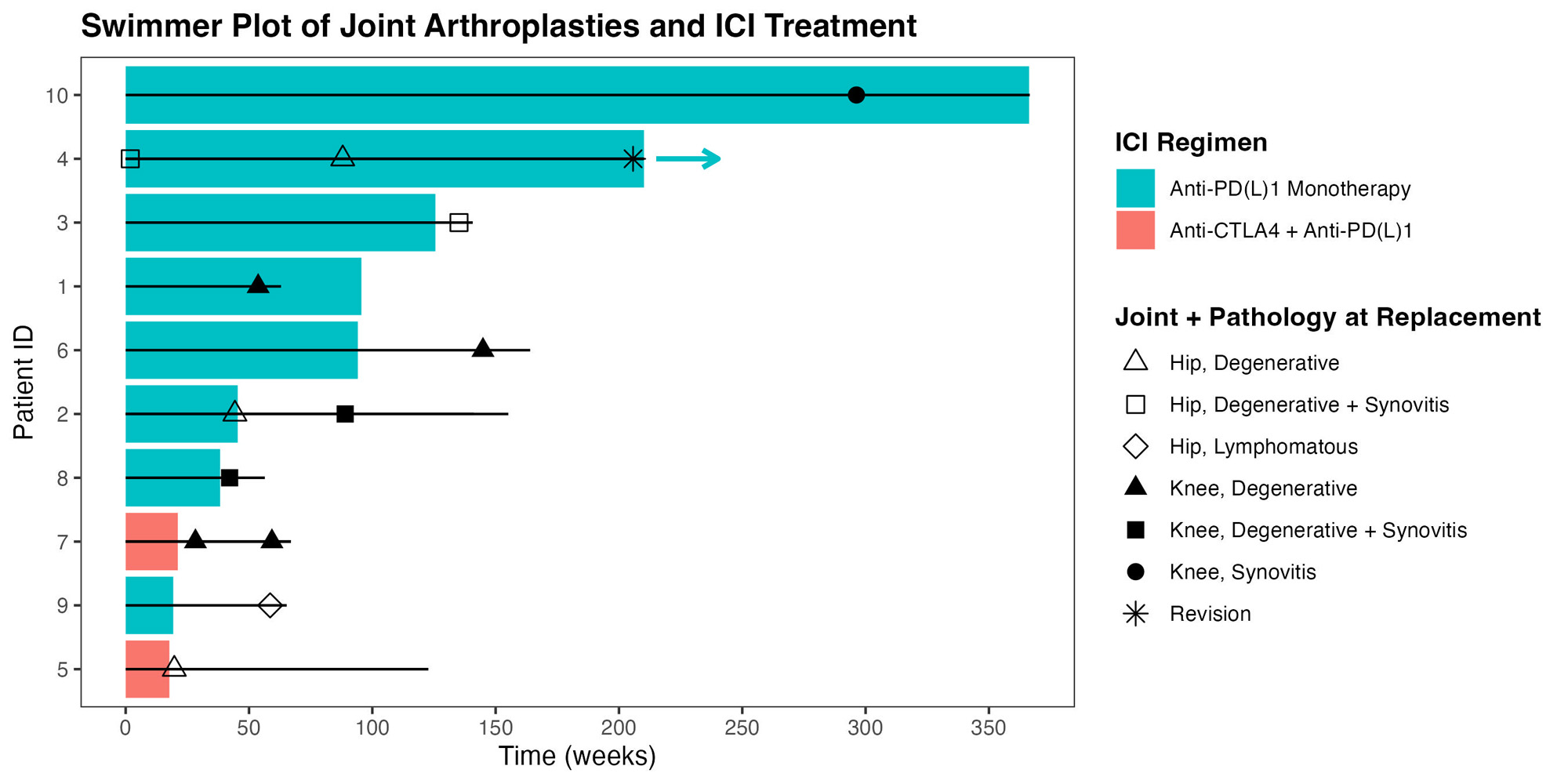
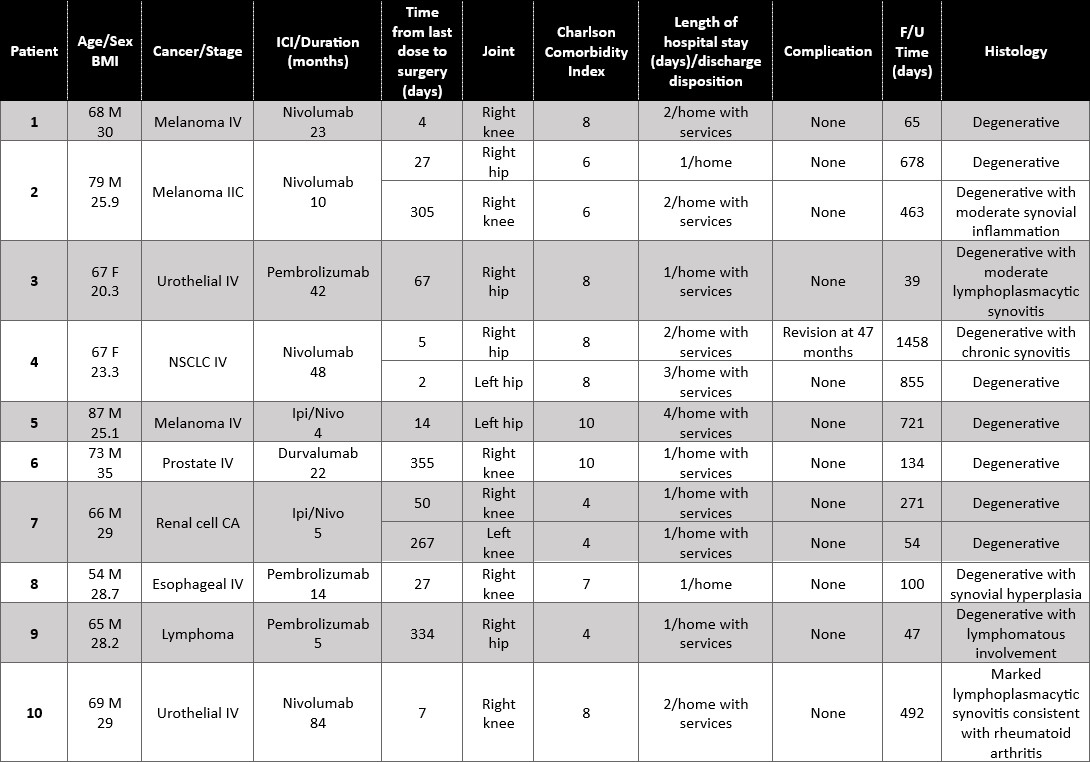
Results: Ten patients who had received ICI treatment underwent 13 native joint arthroplasties (Table 1). Mean age was 70, mean BMI 27.45. Two patients had established ICI-IA. Median duration of ICI therapy was 18 months. Median time from the last dose of ICI to surgery was 27 days. Median duration of follow up was 271 days. There were 6 TKA, 1 unicompartmental knee replacement, and 6 THA (Figure 1). In 12/13, joint replacement surgery was indicated after failing conservative therapy for degenerative joint disease. There were no surgical site infections, wound healing issues, 30-day readmissions, cardiovascular events, or death. All patients were discharged home, most with services. Of the 2 patients with known ICI-IA, one (with known lymphoma) was found to have lymphomatous involvement of an otherwise degenerative hip; the other patient, who had monoarticular ICI-IA and for whom TKR was meant to be curative, had knee histology that showed marked lymphoplasmacytic inflammation resembling rheumatoid arthritis. All other joint explants had degenerative disease, 3 with mild-moderate synovitis. One patient had calcium pyrophosphate crystal deposits. One patient, who has remained on treatment from the time immediately preceding her first THA in 3/2019 until the present, required a revision THA 2/2023. The primary THA used a dual mobility prosthesis and modular dual mobility liner containing cobalt/chromium. Histology of the THA explant at the time of revision showed an immune-mediated inflammatory granulomatous reaction (adverse local tissue reaction), possibly from corrosion at the dual mobility liner, or from neck/stem incompatibility. Serum cobalt level was elevated at 2.7 ucg/L (nv 0.1-0.4). These reactions are uncommon; in one systematic review of arthroplasties using similar components, only 3% of patients had elevated ion levels.1
Conclusion: This single-center retrospective case series demonstrates the relative safety of performing joint arthroplasty in cancer patients treated with ICI. Most patients had degenerative changes with some mild to moderate reactive synovitis. One patient had an immune-mediated reaction leading to early revision; it is plausible that ICI treatment played a role in augmenting the immune response.
1Gkiatas I et al. J Orthop. 2020;21:432-437
To cite this abstract in AMA style:
Elfarra Y, DiCarlo E, Vigdorchik J, Aude C, Ghosh N, Bass A, Chan K. Arthroplasty Outcomes in Immune Checkpoint Inhibitor-Treated Patients: A Single Center Series [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/arthroplasty-outcomes-in-immune-checkpoint-inhibitor-treated-patients-a-single-center-series/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/arthroplasty-outcomes-in-immune-checkpoint-inhibitor-treated-patients-a-single-center-series/