Session Information
Session Type: Poster Session D
Session Time: 1:00PM-3:00PM
Background/Purpose: Monocytes are critical for the pathogenesis of rheumatoid arthritis (RA). However, depletion of circulating monocytes – either classical or non-classical monocytes – is not sufficient to rescue RA in mouse models. Recently, we identified a new cell population of extravascular Ly6c- myeloid cells in the synovial tissue (e.v Syn. Ly6c-) that are distinct from CD64+ macrophages, and required for the development of acute RA-like disease in mouse. Our prior studies suggest that e.v. Syn. Ly6c- in steady state are derived in part from fetal liver that are capable of self-renewal and replenished by circulating monocytes. However, it is unknown whether these ontogenies are associated with different functions over time. A better understanding of the origin of these cells will inform their role in RA and provide avenues for targeted therapies.
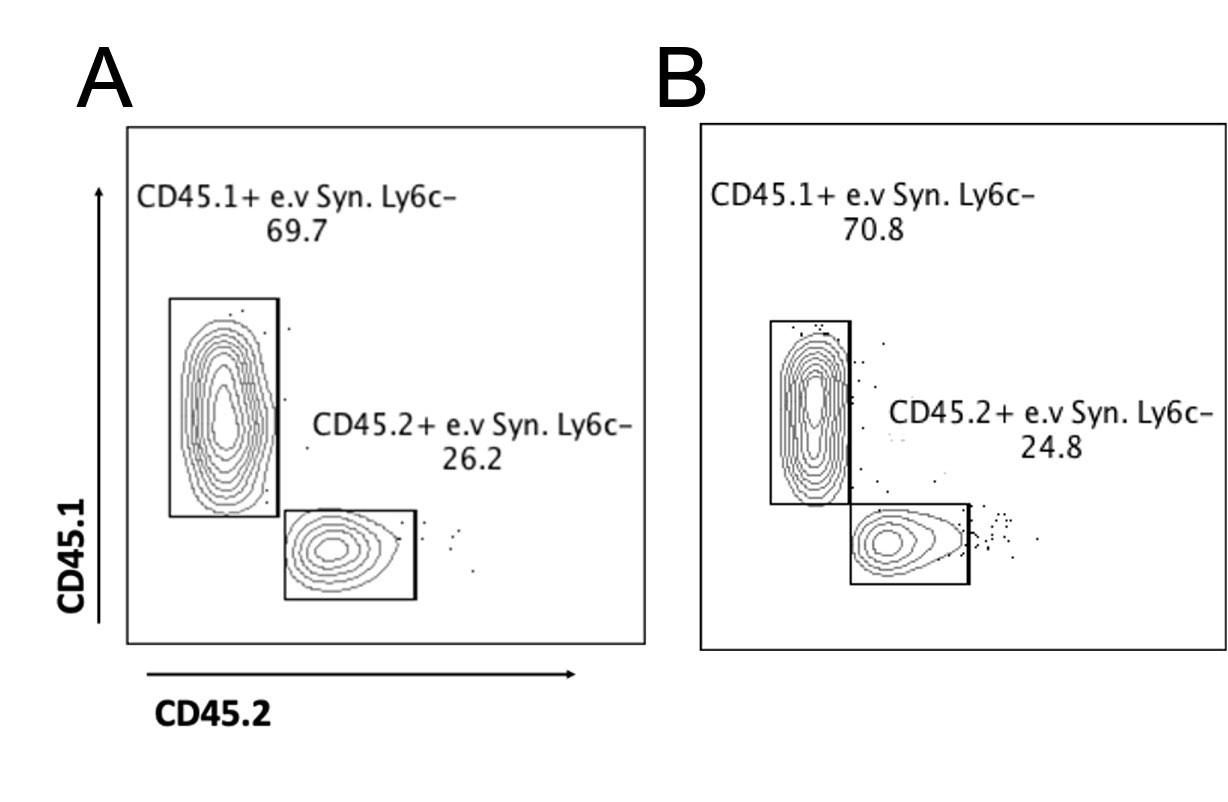
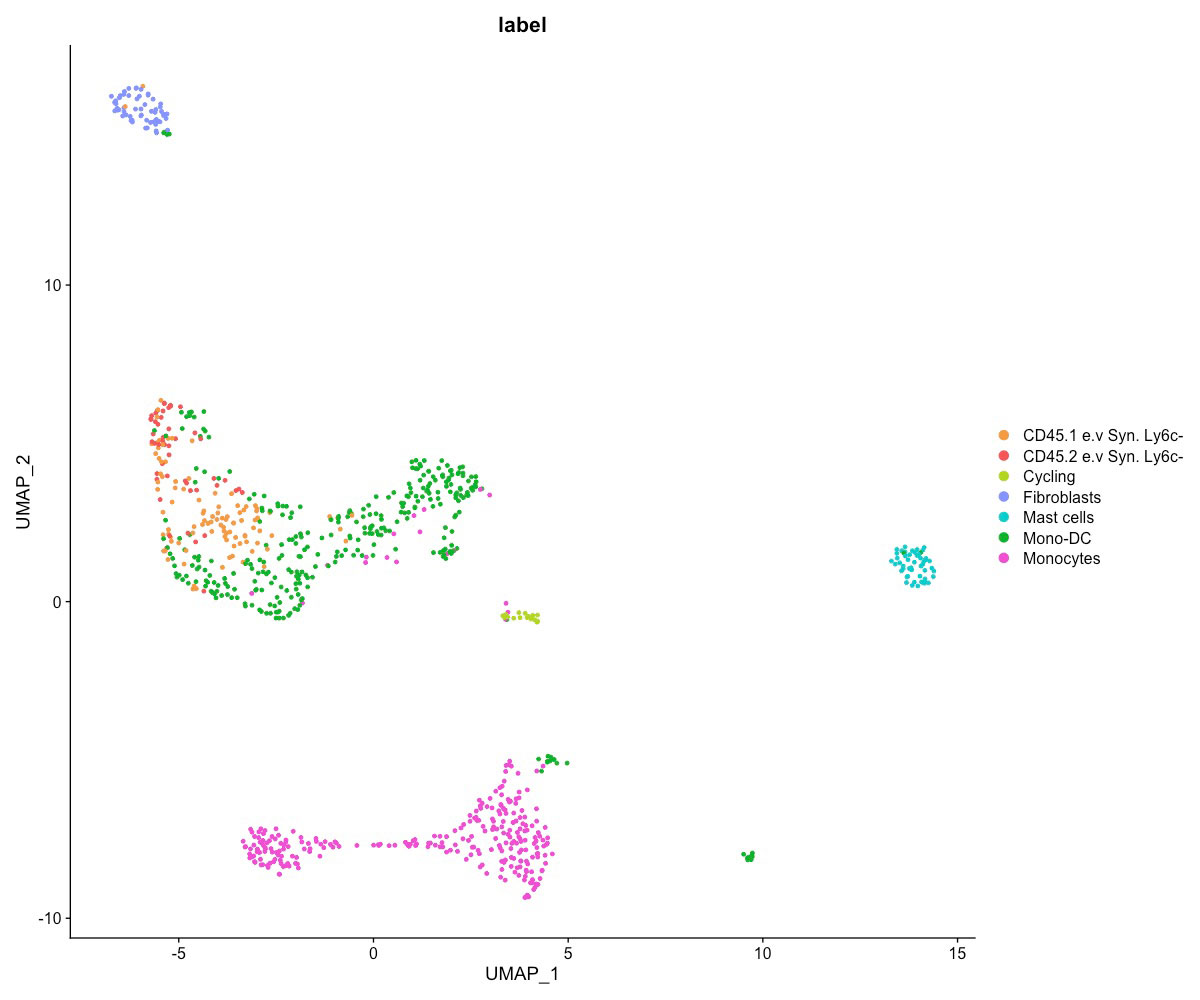
Methods: Bone marrow chimeras (BMC) were generated by transferring CD45.1 cells into CD45.2 mice with ankle shielded to preserve the synovial niche. After 8-month post-chimera, mice were injected intravenously (i.v) with anti-CD45 to label i.v immune cells and sacrificed. Blood was analyzed by flow cytometry to confirm chimerism 90% in circulating cells. Clodronate-liposome (clo-lipo) was injected i.v to disrupt the synovial niche. Ankles were dissected during steady state and 1 day after the clo-lipo injection. Flow cytometry was used to quantify CD45.1 and CD45.2 cells. Cellular Indexing of Transcriptomes and Epitopes by Sequencing (CITE-seq) was performed on sorted CD45+CD11b+MHCII-CD64- cells from the synovium during steady state using 10x Genomics and sequenced on Novaseq 6000. CITE-seq data was processed through the CellRanger v6-1.2 and analyzed with the Seurat package v4.1.0. The circulation-derived and tissue-resident e.v Syn. Ly6c- were distinguished based on the antibody derived tag (ADT) expression of CD45.1 and CD45.2.
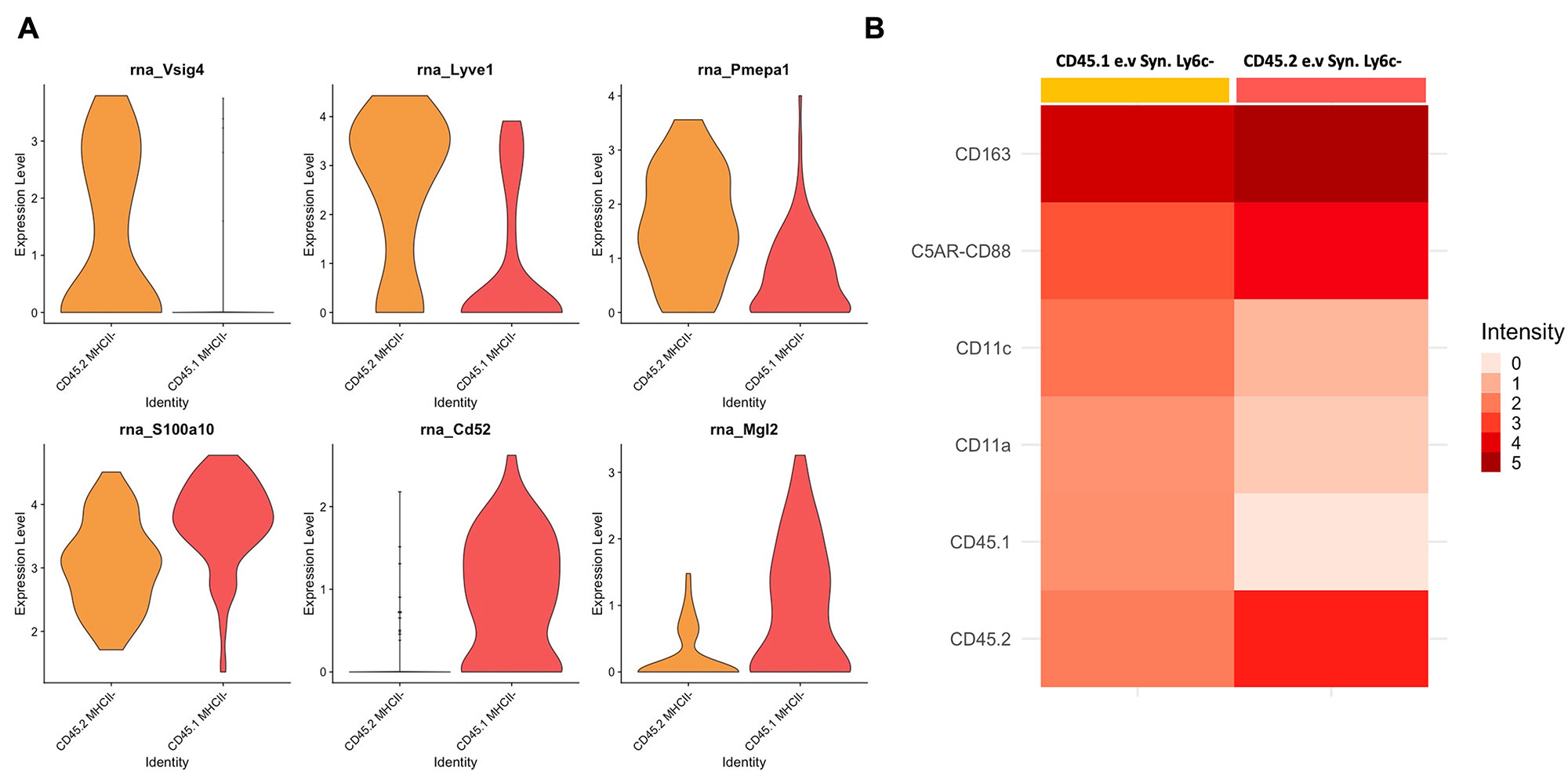
Results: Our flow results suggested that 67% of e.v Syn. Ly6c- cells were circulation-derived (CD45.1+) during steady-state. After the clo-lipo injection, more than 85% of i.v. Syn. Ly6c- cells got depleted and 64% of e.v Syn. Ly6c- cells were circulation-derived. For CITE-seq, the majority of cells were annotated as either circulating monocytes, e.v Syn. Ly6c- cells, or monocyte-derived dendritic cells based on our prior studies. The e.v Syn. Ly6c- clustered into at least 2 subpopulations with distinct transcriptional profiles, and these subpopulations were distinguished by their CD45 status in ADT level. CD45.2 e.v Syn. Ly6c- exhibited higher levels of genes associated with tissue-residence such as Vsig4 and Lyve1, while CD45.1 cells demonstrated increased expression of immune activation genes, such as S100a10 and Cd52. While these populations had similar surface levels of CD43 and CD14, they differed in CD11C and C5AR.
Conclusion: Our prior studies suggest that the expansion of e.v Syn. Ly6c- cells is a key step in the development of RA. Here, we demonstrate that newly recruited e.v Syn. Ly6c- cells derived from circulation demonstrate a distinct transcriptional phenotype from tissue-resident cells. Future studies will determine how the function of circulation-derived and tissue-resident e.v Syn. Ly6c- cells differ in the development of RA.
To cite this abstract in AMA style:
Wang Y, Gurra M, Cuda C, Makinde H, Chen S, Gadhvi G, Dominguez S, Shah C, Winter D, Perlman H. Arthritis-associated Synovial CD64-Ly6c- myeloid Cells Comprise 2 Subpopulations [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/arthritis-associated-synovial-cd64-ly6c-myeloid-cells-comprise-2-subpopulations/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/arthritis-associated-synovial-cd64-ly6c-myeloid-cells-comprise-2-subpopulations/