Session Information
Session Type: Abstract Session
Session Time: 1:00PM-2:30PM
Background/Purpose: In routine care, Danish patients with psoriatic arthritis (PsA) are monitored in DANBIO, a nationwide clinical registry1. Patients enter patient-reported outcomes (PROs) online before consultations in the outpatient clinic. Previously, the PRO measures (PROMs) and physicians’ monitoring have focused on joint inflammation. Since March 2022, new PROMs on selected non-musculoskeletal (non-MSK) manifestations were implemented in DANBIO.
This study aimed to validate the non-MSK PROMs for current dactylitis, current skin and nail psoriasis, and uveitis (since last visit) in patients with PsA.
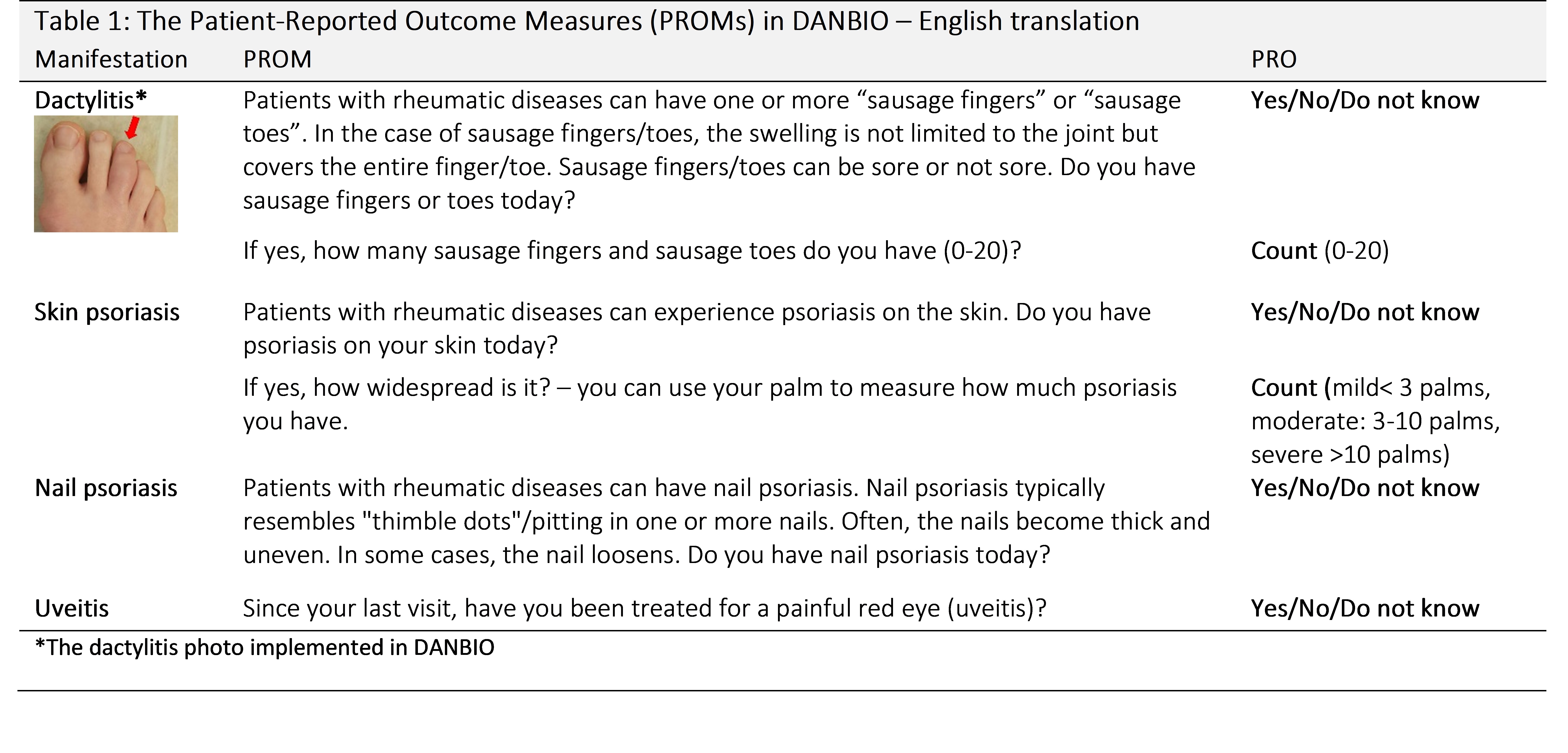
Methods: Cross-sectional study in the rheumatologic outpatient clinic aiming to include 300 patients based on a pre-study power calculation. The patients answered the PROMs with “yes”/”no”/”do not know” and also assessed the extent of psoriasis and number of dactylitis-affected digits (dactylitis count). The patients’ PRO entries were compared to the physician’s assessments, the latter being the gold standard. An interim analysis after recruitment of 134 patients showed that 20% of patients incorrectly reported having dactylitis. Therefore, for subsequent patients, we added a photo of dactylitis to the PROM (PROMs and photo shown in Table 1). From patient number 201 onwards, the physician was blinded to the patients’ responses to investigate confirmation bias.
Sensitivity, specificity, positive (PPV), and negative predictive values (NPV) were calculated. The level of agreement between the physician’s and patients’ dactylitis count was explored by Bland-Altman plot.
Results: We included the planned 300 patients (51% female, mean age=54.0 years (range: 17 to 87 years, SD=14.6)). Current disease-modifying anti-rheumatic drug treatment was: conventional synthetic 43.0%, biologic (mono/combination) 43.3%, and none 13.7%.
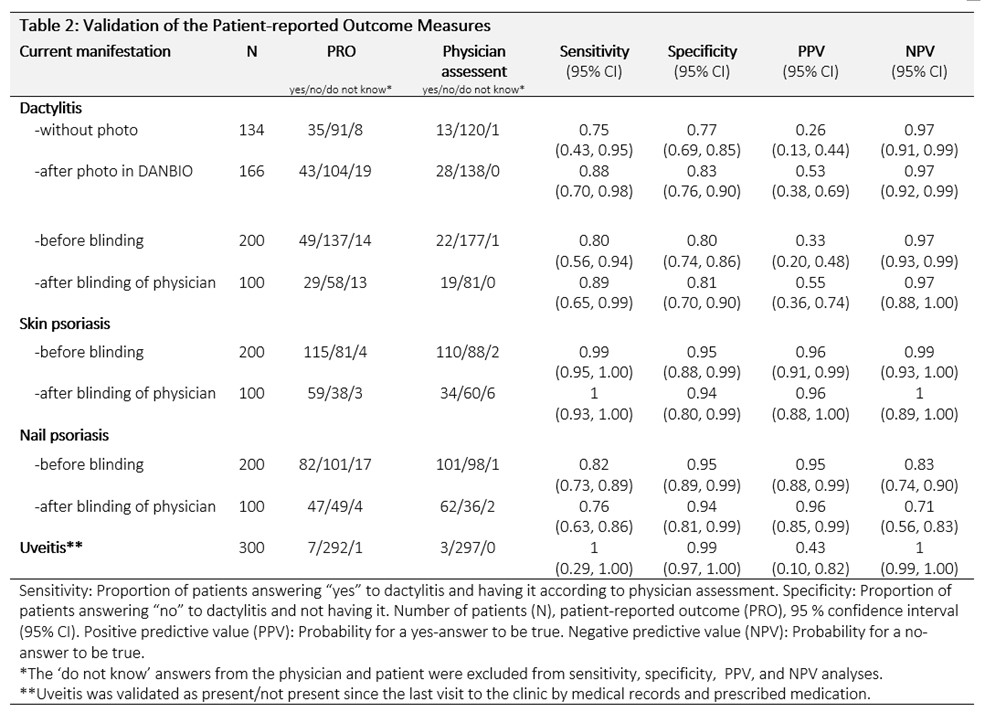
Overall, the physician’s assessment showed that 41 patients had current dactylitis, 164 psoriasis (91.4% mild (< 3 palms), 8% moderate (3-10 palms), 0.6% severe ( >10 palms)), 163 had nail psoriasis, and 3 patients had had uveitis. Validation results are shown in Table 2. For patients without the non-MSK manifestation, agreement between patient and physician was high (high specificities). The agreement for dactylitis improved markedly after implementing a dactylitis photo. The results before and after the blinding of the physician were similar.
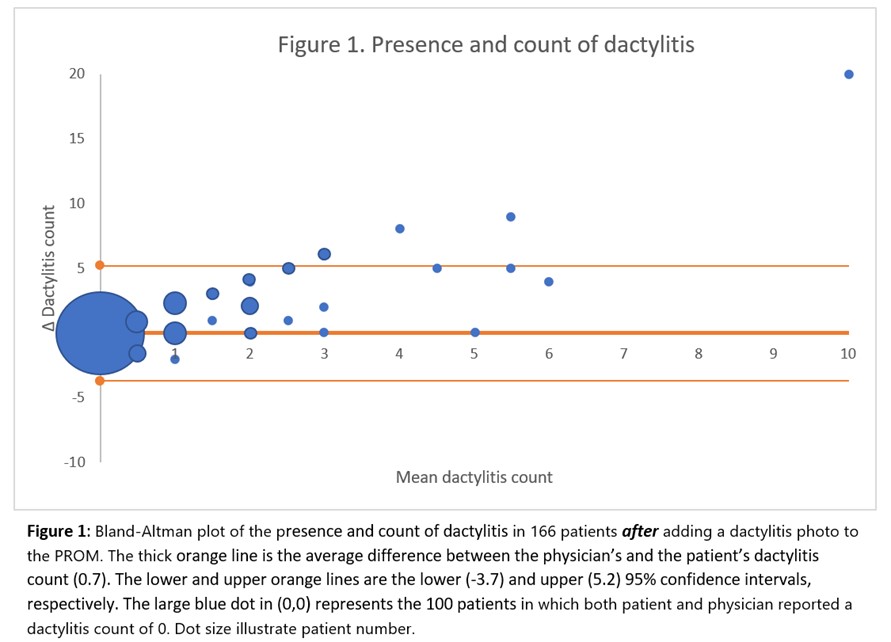
The patient-reported dactylitis count averaged 0.7 units higher than the physician’s assessment (Figure 1). The level of agreement on the presence and extent of psoriasis was 90.1% (data not shown).
Conclusion: We showed that the new PROMs of non-MSK manifestations are valid, and our findings support that patient self-reporting is reliable for monitoring key clinical features of PsA. These findings have implications for successful implementation of the ACR and EULAR recommendations for PsA, stressing the importance of monitoring non-MSK manifestations in routine care.2,3
References
1 Ibfelt EH et al. Clin Epidemiol. 2016 doi: 10.2147/CLEP.S99490
2 Gossec L et al. ARD 2024 doi:10.1136/ard-2024- 225531
3 Singh JA et al. Arthritis Rheumat-ol. 2019 doi: 10.1002/art.40726
Acknowledgment
Image courtesy of Dr. Orbai, Johns Hopkins, USA
To cite this abstract in AMA style:
Nielung L, Hetland M, Skov L, Frandsen H, Dreyer L, Glintborg B. Are Patient-reported Outcomes for Dactylitis, Uveitis, Psoriatic Skin and Nail Disease Valid in Patients with Psoriatic Arthritis? [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/are-patient-reported-outcomes-for-dactylitis-uveitis-psoriatic-skin-and-nail-disease-valid-in-patients-with-psoriatic-arthritis/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/are-patient-reported-outcomes-for-dactylitis-uveitis-psoriatic-skin-and-nail-disease-valid-in-patients-with-psoriatic-arthritis/