Session Information
Date: Monday, November 13, 2023
Title: (1100–1123) Metabolic & Crystal Arthropathies – Basic & Clinical Science Poster II
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: The uric acid transporter inhibitor (URAT1) is responsible for the reabsorption of filtered uric acid from the renal tubular lumen. Uricosuric agents inhibit URAT1 consequently lowers serum urate (sUA). With previous uricosurics, bolus dumping of drug to the target site caused spontaneous accumulation of uric acid in renal tubules, which posed a potential influx of uric acid at high concentration leading to renal toxicity.1 AR882 is a potent and selective URAT1 transporter inhibitor in late-phase clinical development. To illustrate the renal safety of AR882 the uric acid excretion profiles across multiple studies were collected to assess the uric acid excretion amount and rate following once-daily dosing.
Methods: In phase 1 healthy subject studies, uric acid excretion profiles were collected following 25, 50 and 75 mg once-daily doses for 10 days in a multiple-ascending dose study (n=24) and following 75 mg once-daily for 14 days (n=16) with every 2-hour collection intervals in a renal impairment study. In a phase 2b study, urine samples were collected at every 2-hour intervals up to 8 hours post-dose from 25 gout patients to evaluate the excretion amount, rate and fractional excretion of uric acid following 8 weeks of once-daily dosing at 50 and 75 mg.
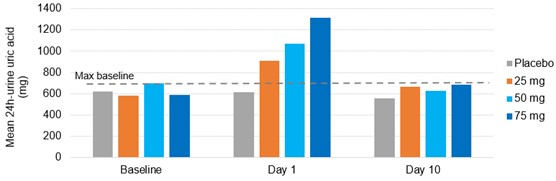
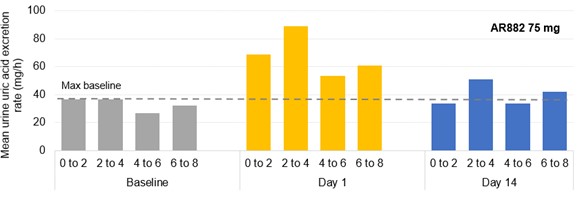
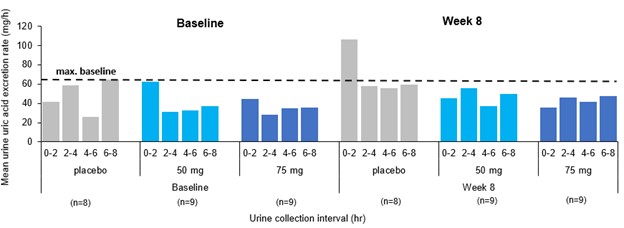
Results: In phase 1 studies, AR882 showed a relatively flat exposure profile with a slow rise to peak concentrations and slow decline over time. The fraction of dose excreted into tubules was evenly distributed across the day, mirroring the PK profile and supported its sustained sUA reduction and long-lasting URAT1 inhibitory pattern. Following 10 or 14 days of dosing, AR882 showed similar excretion amount to baseline (Figure 1) and similar intra-day excretion rate (Figure 2). In gout patients, the maximum uric acid excretion rate at baseline was under approximately 60 mg/hr. After 8-week treatment, AR882 at either 50 mg or 75 mg showed consistently low excretion rate across 8 hours post-dose which was similar to baseline or under the maximum baseline value (Figure 3) while maintaining significant uric acid lowering effect.
Conclusion: With its unique, slower elimination in PK, AR882 maintains a smooth intra-day uric acid excretion profile that was similar to baseline or to that in placebo patients, resulting in a lasting inhibitory effect without any high concentration of uric acid influx in the kidney tubules. The lack of daily transient increase in uric acid excretion confirms AR882, as a new uricosuric agent, possessing a favorable and safe renal profile over existing and other approved uricosuric agents.
Reference: 1. Sanchez-Niño MD, Zheng-Lin B, Valiño-Rivas L, et al. Lesinurad: what the nephrologist should know. Clin Kidney J. 2017 Oct;10(5):679-687.
To cite this abstract in AMA style:
Shen Z, Polvent E, Morris s, Yan R, Yan S, Keenan R, Yeh L. AR882, a Potent Uricosuric Agent, Shows Favorable Uric Acid Excretion Profile Following Multiple Doses [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/ar882-a-potent-uricosuric-agent-shows-favorable-uric-acid-excretion-profile-following-multiple-doses/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/ar882-a-potent-uricosuric-agent-shows-favorable-uric-acid-excretion-profile-following-multiple-doses/