Session Information
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: AR882 is a potent and selective uric acid transporter 1 (URAT1) inhibitor under development for the treatment of hyperuricemia or gout. AR882 exhibited linear pharmacokinetic (PK) properties, dose-dependent serum urate (sUA) lowering effect and was well tolerated in a single ascending dose (SAD) study in humans. A multiple ascending dose (MAD) study was therefore conducted in healthy subjects to evaluate the PK, pharmacodynamic (PD) and safety profiles of AR882 following once-daily (QD) doses for 10 days.
Methods: In this randomized, double-blind, placebo-controlled MAD study, 30 healthy male volunteers received AR882 oral capsules under fasted conditions at 25, 50, or 75 mg (ten subjects per group [8 active and 2 placebo]). On Day -1, Day 1 and Day 10, serial blood samples were collected for measurement of AR882 plasma concentrations and sUA levels for PK/PD assessments. Urine samples were collected at 6- to 12-hour intervals for assessment of uric acid excretion. Adverse events, laboratory safety tests, vital signs, and electrocardiograms were collected throughout the study.
Results: Following QD administration, AR882 plasma levels exhibited dose proportional increases between 25 and 75 mg. AR882 exposures were well below those observed at the no-observed-adverse-effect level (NOAEL) in preclinical studies. The median Tmax ranged between 1.5 and 3.5 hours postdose, and accumulation in Cmax and AUC was between 30-50%. On Day 10 predose, AR882 showed significant PD effects by reducing sUA concentrations by 41%, 58%, and 65%, respectively, following 25, 50, and 75 mg doses. Steady-state sUA lowering effect was achieved after approximately 5 days. Higher amounts of uric acid were eliminated through urine on Day 1 at all collection intervals. On subsequent days, daily urine uric acid gradually decreased. Fractional excretion of uric acid (FEUA) showed dose-dependent increases on Day 1 and Day 10, consistent with reduction in serum urate levels. AR882 was well tolerated at all doses tested. All AEs were mild, there were no discontinuations due to AEs, and no serious adverse events (SAEs) were reported. There were no clinically significant laboratory or ECG abnormalities noted.
Conclusion: Following once-daily doses over a 10-day treatment period, AR882 exhibited significant sUA lowering effects at all doses tested (58% at Day 10 predose and up to 67% maximum intraday reduction at 50 mg). These results support the potential utility of AR882 when given once-daily to treat hyperuricemia or gout. Studies in patients are underway.
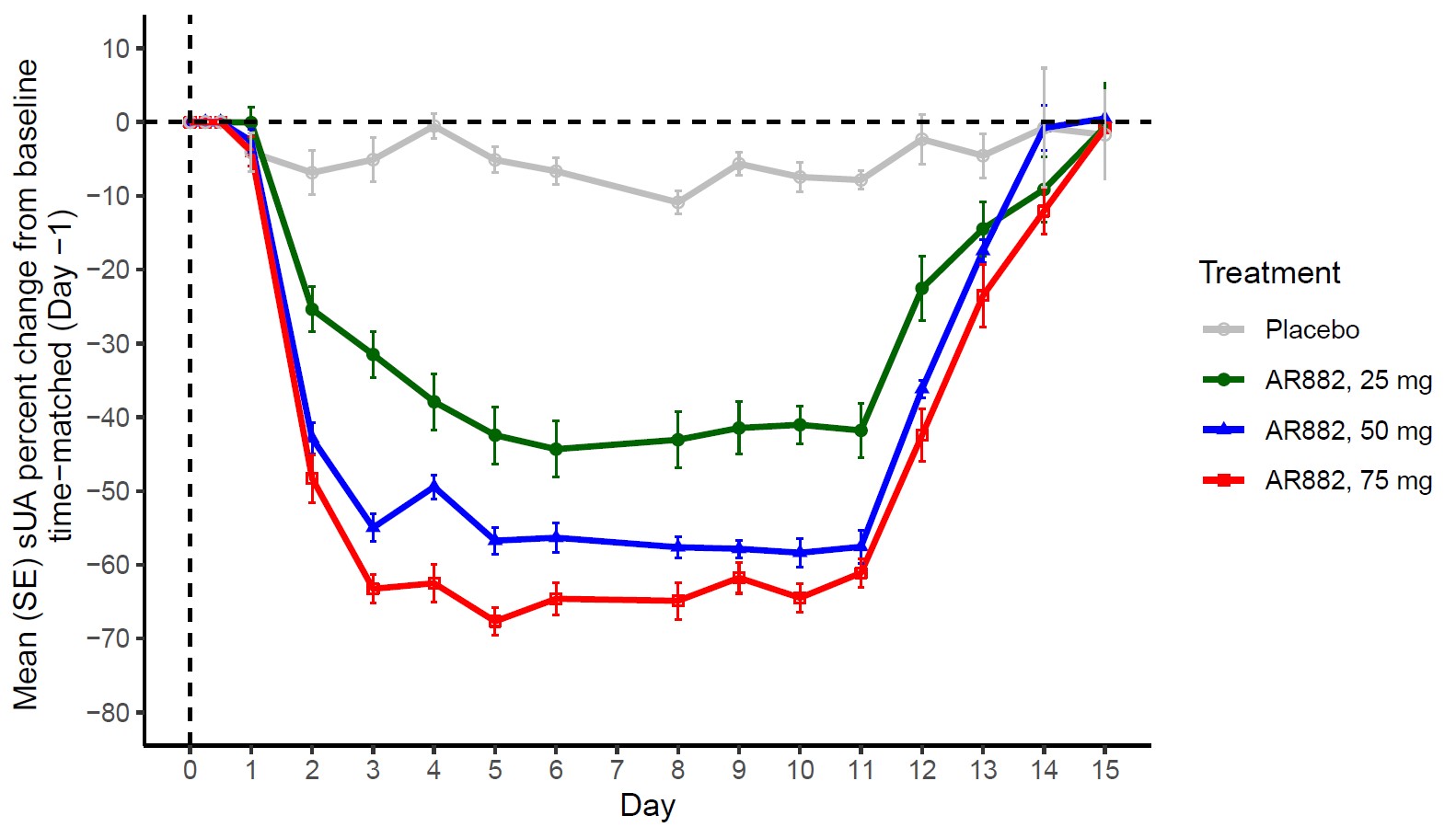 Figure: Mean Serum Urate Concentrations: Percent Change from Baseline Following Once-Daily Oral Doses of AR882
Figure: Mean Serum Urate Concentrations: Percent Change from Baseline Following Once-Daily Oral Doses of AR882
To cite this abstract in AMA style:
Shen Z, Polvent E, Hingorani V, Clouser-Roche A, Mikelatis C, Yan R, Yan S, Yeh L. AR882, a Potent and Selective Uricosuric Agent, Significantly Reduced Serum Urate Levels Following Multiple Ascending Once-Daily Doses in Healthy Subject Volunteers [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/ar882-a-potent-and-selective-uricosuric-agent-significantly-reduced-serum-urate-levels-following-multiple-ascending-once-daily-doses-in-healthy-subject-volunteers/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/ar882-a-potent-and-selective-uricosuric-agent-significantly-reduced-serum-urate-levels-following-multiple-ascending-once-daily-doses-in-healthy-subject-volunteers/
