Session Information
Date: Monday, November 13, 2023
Title: Abstracts: Spondyloarthritis Including Psoriatic Arthritis – Treatment II: PsA
Session Type: Abstract Session
Session Time: 4:00PM-5:30PM
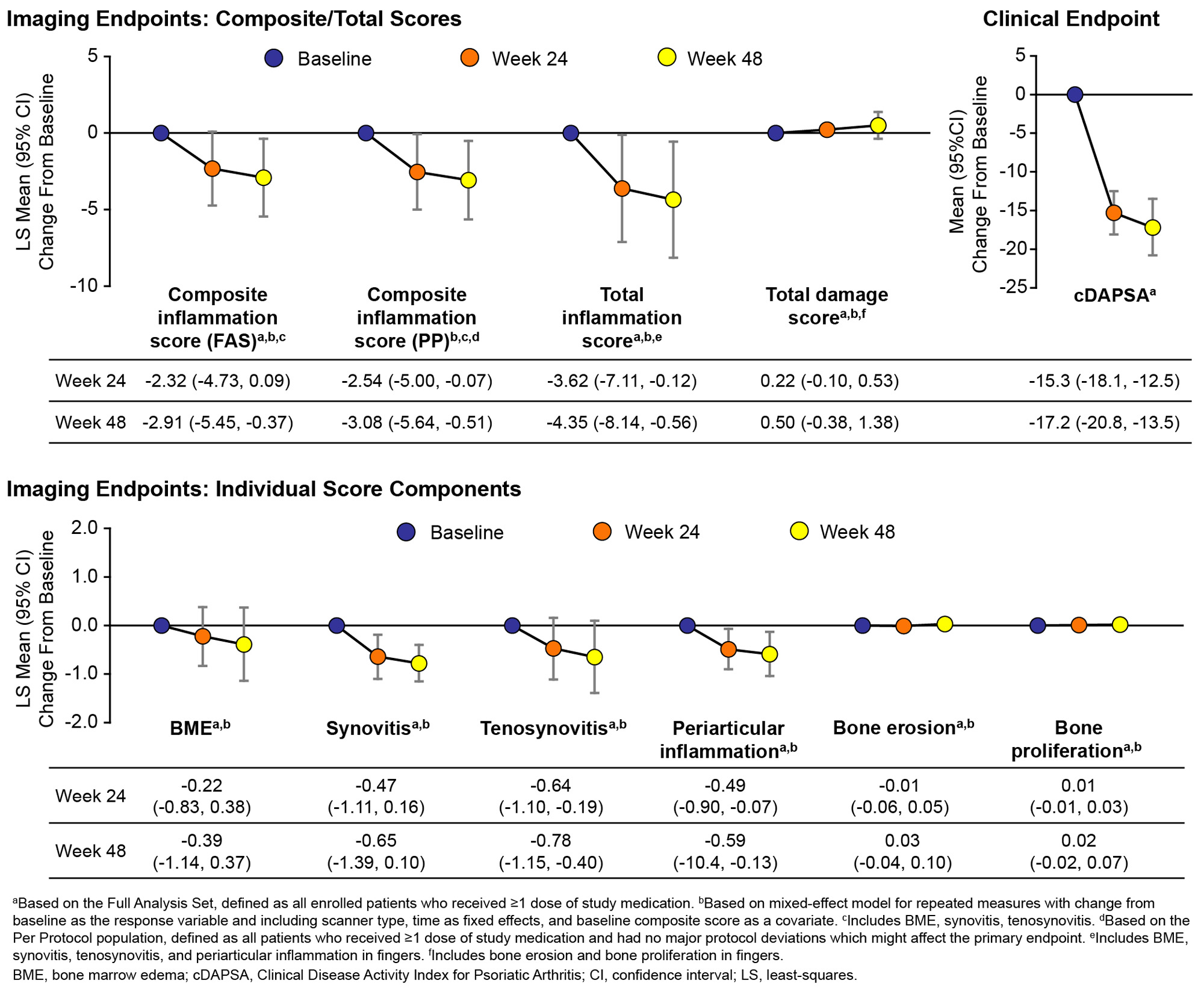
Background/Purpose: PsA is characterized by inflammatory arthritis, enthesitis, dactylitis, and spondylitis. Apremilast (APR) is an oral immunomodulating phosphodiesterase-4 inhibitor approved for the treatment of PsA. Here, we evaluate the efficacy of APR 30 mg BID on inflammation measured by a dedicated MRI of the hand.
Methods: MOSAIC (NCT03783026) was a phase 4, multicenter, single-arm, open-label study in patients (pts) with active PsA (≥3 months but ≤5 years since diagnosis, meeting CASPAR criteria) evaluating APR as monotherapy or in combination with stable MTX. Pts were treated with APR for 48 weeks (wk) and had contrast-enhanced MRI of the hand performed at baseline (BL), Wk 24, and Wk 48. All images were read and adjudicated by 2 experienced readers blinded to clinical information and time of acquisition. The primary endpoint was change from BL in the composite score of hand bone marrow edema (BME), synovitis, and tenosynovitis in fingers 2–5, as assessed by the PsA MRI Score (PsAMRIS) at Wk 24. Total inflammation score, comprised of BME, synovitis, tenosynovitis, and periarticular inflammation in fingers, was also assessed. Structural progression determined by bone erosion and proliferation in fingers 2–5 was assessed by the total hand damage score. Subgroup analyses based on BL disease activity as measured by Clinical Disease Activity Index for PsA (cDAPSA) were performed for key endpoints.
Results: A total of 122 pts enrolled and received APR (mean age, 47 y; 55% female; and mean PsA duration, 1.9 y). The Full Analysis Set (FAS) included 98 pts evaluable for the primary endpoint and 94 were included in the per protocol (PP) set. The least-squares (LS) mean (95% CI) change from BL in the PsAMRIS (FAS) was -2.32 (-4.73, 0.09) at Wk 24 and -2.91 (-5.45, -0.37) at Wk 48 (Figure 1). In the PP set, the LS mean (95% CI) change from BL in the PsAMRIS at Wks 24 and 48 showed a significant reduction of disease activity (Figure 1). Significant improvements from BL were seen in total inflammation scores in the FAS (Figure 1). The total hand damage score showed no significant change from BL to Wk 48 (Figure 1). Pts also experienced significant improvements from BL in cDAPSA at Wks 24 and 48 (Figure 1). Subgroup analyses based on BL disease activity showed significant improvements from BL in inflammation in pts with moderate disease activity (ModDA;cDAPSA >13 to ≤27) and no significant change from BL in total damage. Though it was insignificant, pts with high disease activity (HDA; cDAPSA >27) did have improvement from BL in inflammation indices (Figure 2). No new safety signals were identified.
Conclusion: Pts with PsA treated with APR had improvements in both clinical indices and objective MRI indices of inflammation assessed by PsAMRIS in the target hand at Wk 24 and Wk 48, confirming an effect of APR on clinical and inflammatory manifestations of PsA. Pts with ModDA seemed to have greater improvement from BL in MRI inflammation scores than pts with HDA. No significant structural progression was observed. These results offer important insights on the effect of APR in PsA and highlight the value of using MRI and PsAMRIS as measures of inflammatory disease activity and change following treatment.
To cite this abstract in AMA style:
Østergaard M, Maksymowych W, Boesen M, Lambert R, Valenzuela G, Bubb M, Kubassova O, Reddy J, Colgan S, Klyachkin Y, Deignan C, Tang L, Paris M, Mease P. Apremilast Reduces Inflammation as Measured by MRI of the Hand in Patients with Psoriatic Arthritis: Primary Results from the Phase 4 MOSAIC Study [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/apremilast-reduces-inflammation-as-measured-by-mri-of-the-hand-in-patients-with-psoriatic-arthritis-primary-results-from-the-phase-4-mosaic-study/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/apremilast-reduces-inflammation-as-measured-by-mri-of-the-hand-in-patients-with-psoriatic-arthritis-primary-results-from-the-phase-4-mosaic-study/