Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Janus kinase inhibitors (JAKi) have been proposed as a treatment for idiopathic inflammatory myopathies to target increased interferon signaling. Predominantly retrospective reports have demonstrated effectiveness of JAKi in refractory juvenile dermatomyositis (JDM). However, JAKi remain an off-label treatment for JDM and there may be variation in use worldwide. We conducted an international survey to investigate approaches to use of JAKi by pediatric rheumatology providers in JDM.
Methods: The Childhood Arthritis and Rheumatology Research Alliance (CARRA) JDM Therapeutics workgroup and core members of the Paediatric Rheumatology European Society (PReS) JDM working party devised an electronic survey to assess the use of JAKi in JDM. CARRA and PReS members were invited by email to complete the survey.
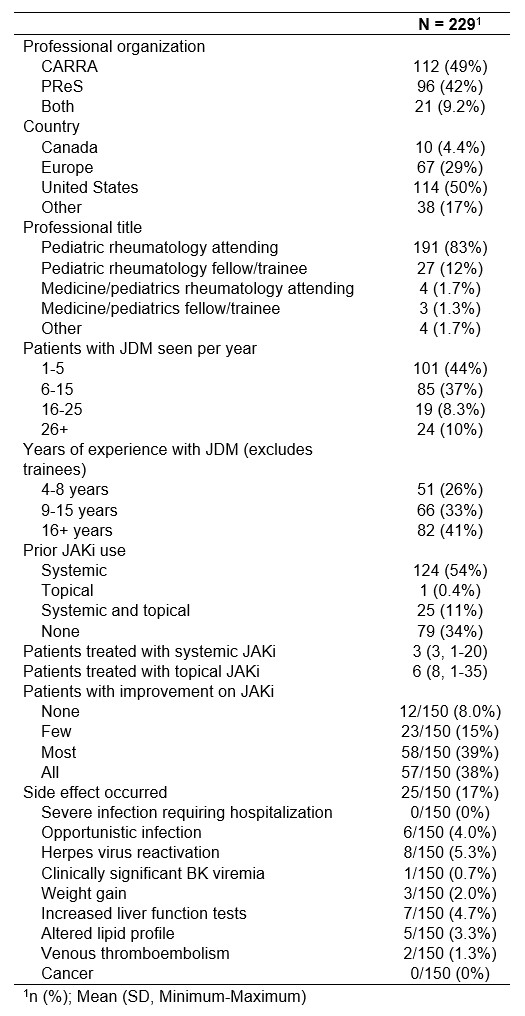
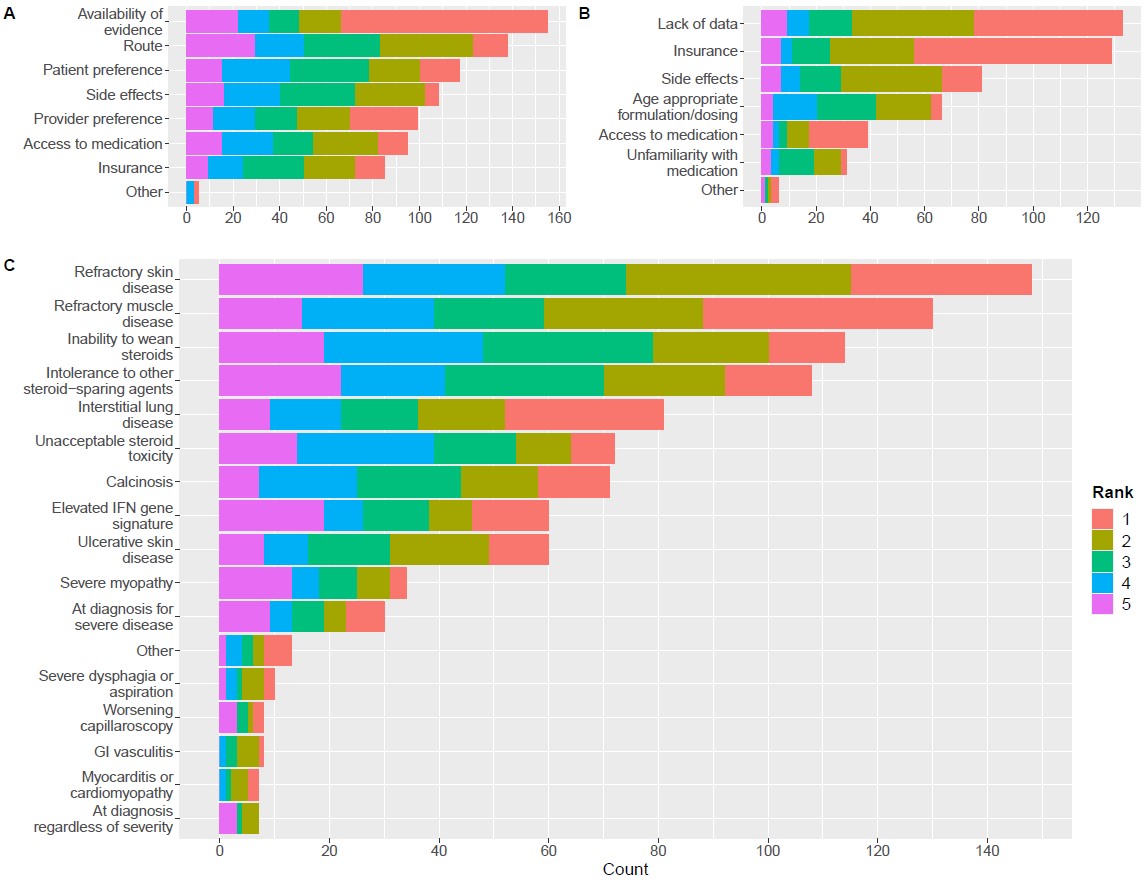
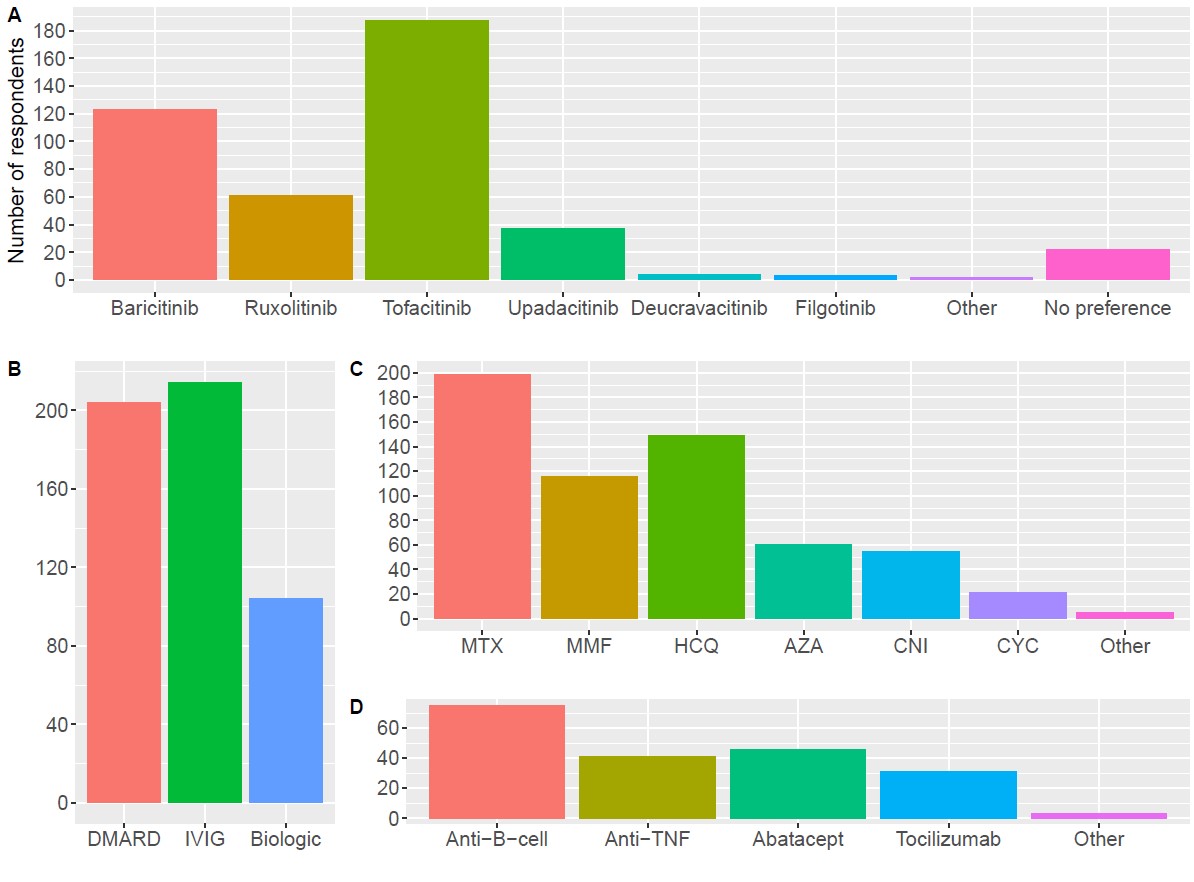
Results: 229 respondents (18% of total recipients, 29% [130 of 453] from CARRA and 12% [99 of 817] from PReS) were included (Table 1). 83% of respondents were attending pediatric rheumatologists. 50% were from the US, and 29% were from Europe. 66% (n=150) had used JAKi for JDM. Among them, 77% noted clinical improvement in most or all patients. Side effects were reported by 17%, including opportunistic infection (4%), herpes virus reactivation (5.3%), increased liver function tests (4.7%), and venous thromboembolism (1.3%). No cancers were reported. The most highly ranked considerations for starting JAKi included available evidence, route of administration, and patient preference (Figure 1). The highest ranked perceived barriers to use of JAKi were lack of clinical data and inability to obtain insurance approval. Of note, insurance approval was indicated as a barrier by 75% of US respondents compared to 33% from Europe. The highest ranked clinical indications for starting JAKi were refractory skin disease, refractory muscle disease, inability to wean steroids, intolerance to other steroid-sparing agents, and interstitial lung disease. Among available JAKi, tofacitinib and baricitinib were the most frequently considered (Figure 2). Approximately 90% of respondents indicated that they would consider using a disease-modifying antirheumatic drug (DMARD) or intravenous immunoglobulin in combination with systemic JAKi. Only 45% would consider using a biologic in combination. Methotrexate, hydroxychloroquine, and mycophenolate were the DMARDs most considered for use in combination with systemic JAKi. Anti-B-cell agents followed by abatacept, anti-TNF, and tocilizumab were the biologics most considered for use in combination with systemic JAKi.
Conclusion: Pediatric rheumatologists use JAKi as an off-label treatment for refractory disease in JDM. Most providers who have used JAKi have seen clinical improvement in their patients. Major barriers to the use of JAKi include lack of clinical data and insurance coverage. Clinical trials are needed to provide better data on the efficacy and safety of JAKi.
Acknowledgements: The authors wish to acknowledge CARRA and the ongoing Arthritis Foundation financial support of CARRA. Intramural Research Program of the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the NIH (AR041215).
To cite this abstract in AMA style:
Datyner E, Nicolai R, Rosina S, Hamilton A, Ardalan K, Bader-Meunier B, Brown A, Campanilho-Marques R, Jansen M, Kim S, Lang B, McCann L, Sanner H, Wilkinson M, Yi B, Kim H, Sherman M, Tarvin S, Papadopoulou C. Approach to Janus Kinase Inhibition for Juvenile Dermatomyositis Among Childhood Arthritis and Rheumatology Research Alliance (CARRA) and Paediatric Rheumatology European Society (PReS) Providers [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/approach-to-janus-kinase-inhibition-for-juvenile-dermatomyositis-among-childhood-arthritis-and-rheumatology-research-alliance-carra-and-paediatric-rheumatology-european-society-pres-providers/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/approach-to-janus-kinase-inhibition-for-juvenile-dermatomyositis-among-childhood-arthritis-and-rheumatology-research-alliance-carra-and-paediatric-rheumatology-european-society-pres-providers/