Session Information
Session Type: Poster Session C
Session Time: 1:00PM-3:00PM
Background/Purpose: Collection and use of rheumatoid arthritis (RA) disease activity and functional status outcome measures facilitates treating-to-target and shared decision-making. Although guidelines recommend regular use of these measures and most rheumatologists agree on their value, implementation in a busy clinical practice can be challenging. Indeed, collection of these measures varies widely in RISE registry practices. In this study, we used the Consolidated Framework for Implementation Research (CFIR) as an evaluation framework to study practices that reliably collect RA measures.
Methods: We used purposive sampling among RISE practices with a high proportion of patients with documented RA outcome measures (top quartile across all RISE practices). Because academic medical centers (AMCs) are under-represented in RISE, we also sampled several high-performing AMCs. A rheumatologist and key staff from each practice involved in RA measure collection were invited for a group interview. A semi-structured interview guide was developed using concepts from the CFIR framework. Recorded interviews were transcribed, and deductive and inductive thematic analysis was used to identify key themes. We organized themes and sub-themes into components within CFIR domains.
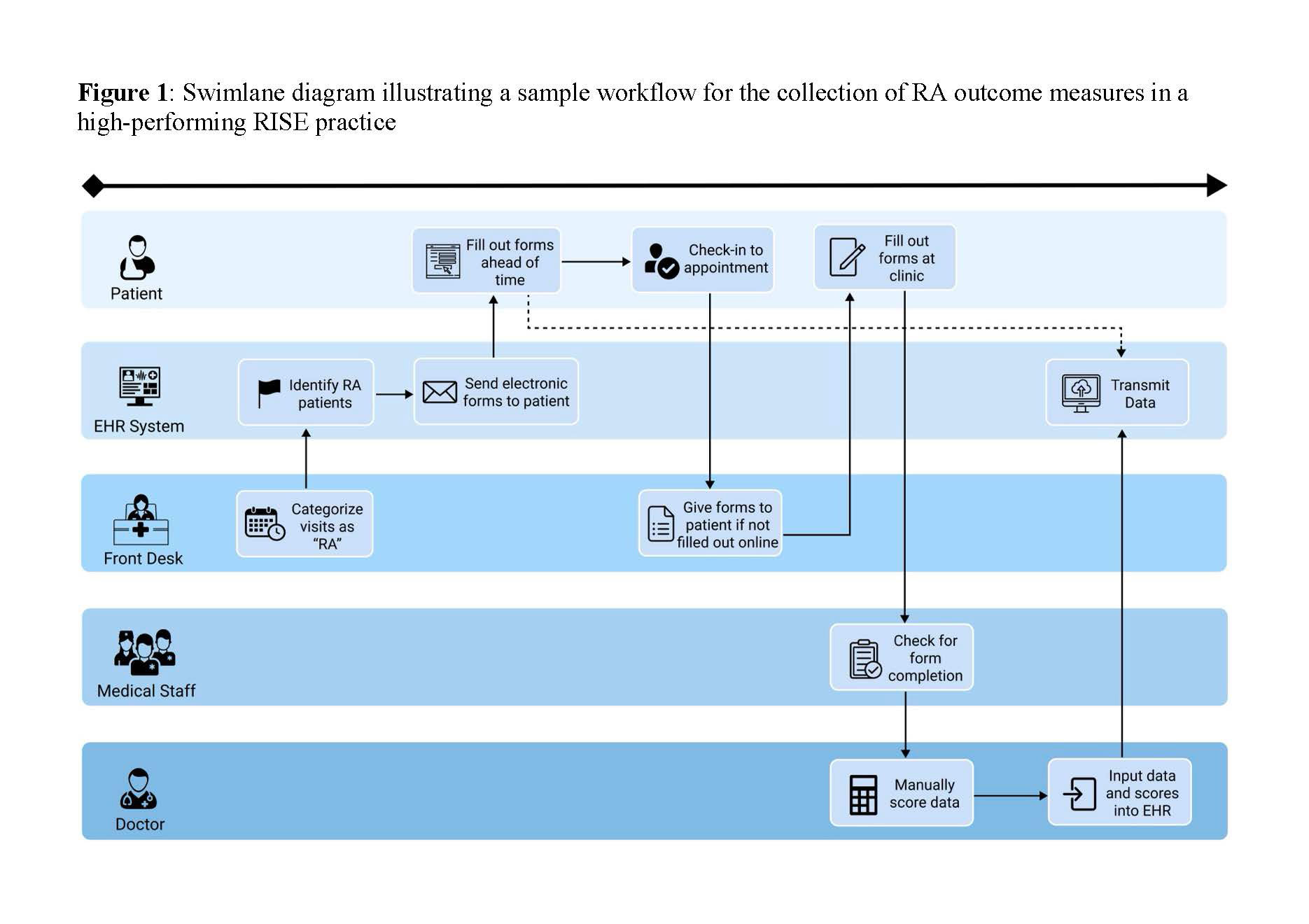
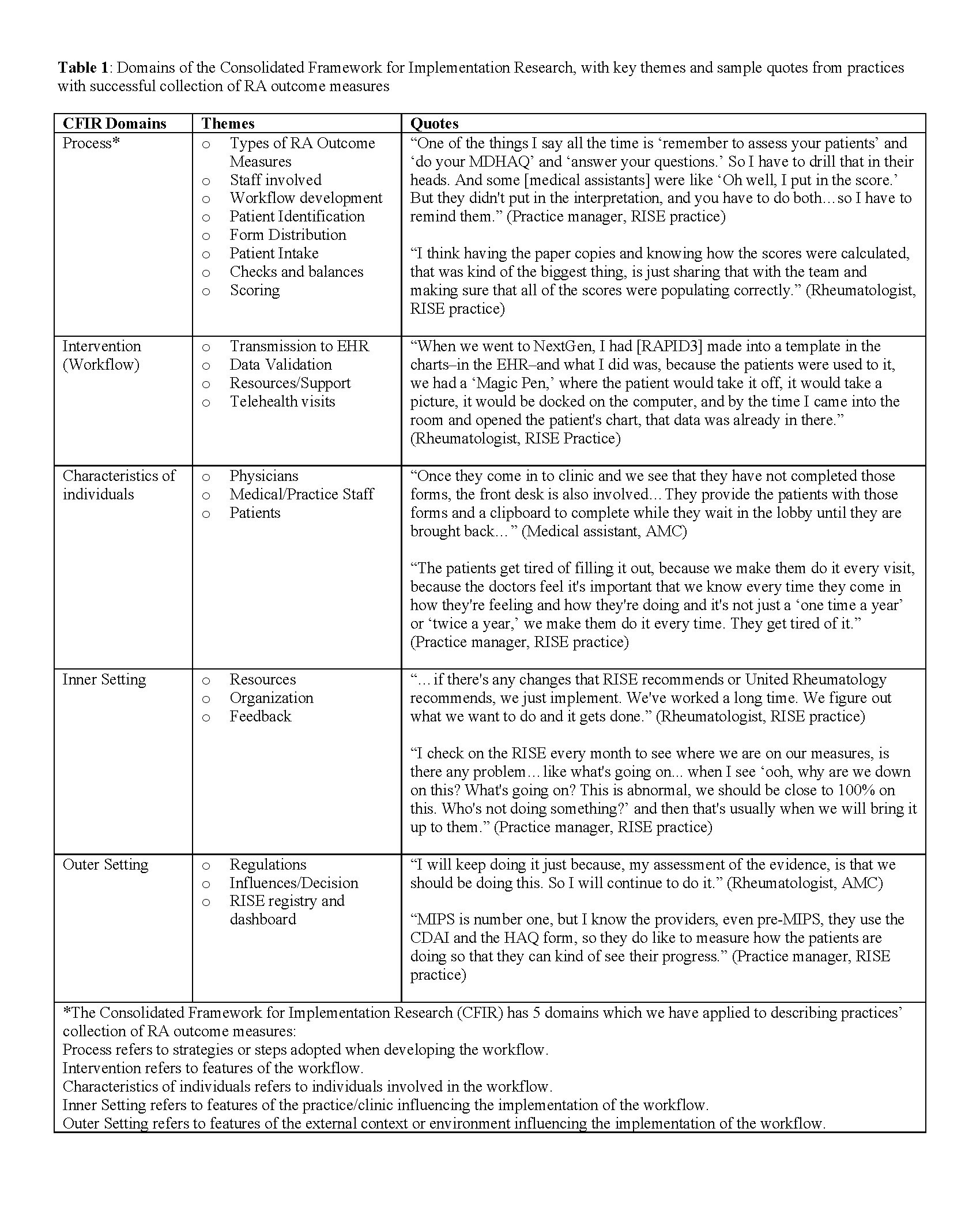
Results: We conducted 29 interviews across 10 RISE practices and 5 AMCs (16 rheumatologists, 6 medical assistants, 5 practice managers, 1 nurse practitioner, and 1 IT specialist). Common themes in successful implementation of RA measure collection were discussed by participants in the following CFIR domains (Table 1): 1) Process: practices had clearly defined workflows for collecting RA outcomes and well-defined roles for staff and physicians in collecting RA measure information (Figure 1); 2)
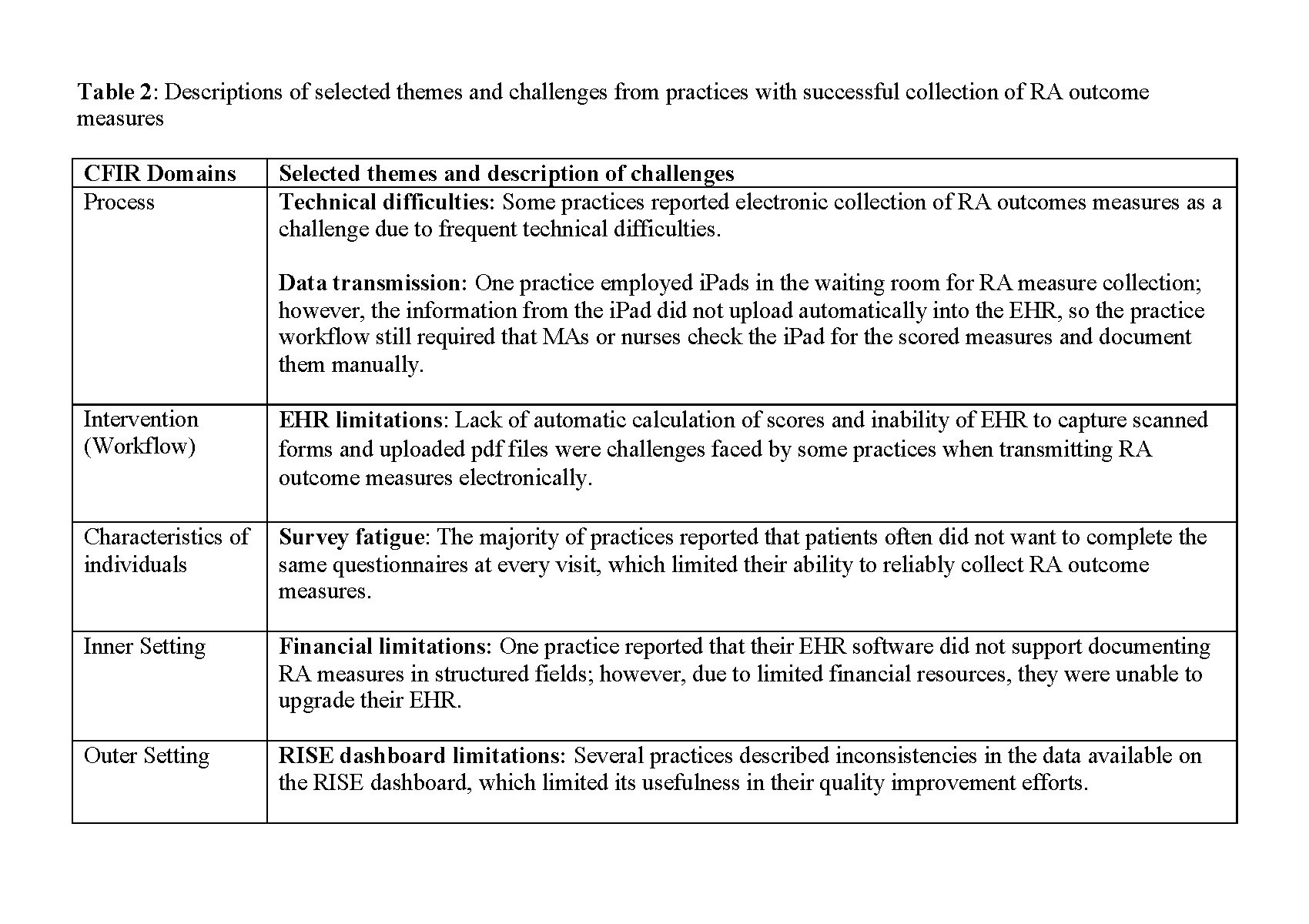
Intervention: practices tailored RA measure collection procedures to their clinical context, employing everything from simple paper forms to digital applications supplied by an outside vendor or within the EHR; 3) Characteristics of individuals: nearly all participants emphasized that clinic culture was important to their success. The idea of “checks and balances,” or ensuring multiple staff members are responsible for collection of RA measures, was common; 4) Inner setting: capacity for rapid adjustments to workflows was common, with many practices reporting the ability to implement changes quickly as needed; 5) Outer setting: although external quality reporting programs necessitated measure collection, most respondents relayed that this was rarely the primary driver of their performance. Instead, rheumatologists felt motivated to provide high quality, evidence-based care to patients. Despite their success in collecting RA measures, respondents also identified a number of challenges (Table 2).
Conclusion: Rheumatology practices that successfully collect RA measures reported having well-defined workflows, a culture of accountability, and being motivated by patients and evidence as opposed to external factors such as pay-for performance programs. Next steps include using these qualitative findings to build a quality improvement toolkit to disseminate to rheumatology practices nationwide.
To cite this abstract in AMA style:
Jacobsohn L, Nasrallah C, Young C, Schmajuk G, Yazdany J. Application of the Consolidated Framework for Implementation Research (CFIR) to Study Top-Performing Practices in the RISE Registry [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/application-of-the-consolidated-framework-for-implementation-research-cfir-to-study-top-performing-practices-in-the-rise-registry/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/application-of-the-consolidated-framework-for-implementation-research-cfir-to-study-top-performing-practices-in-the-rise-registry/