Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Anti-Beta-2-glycoprotein 1 antibody (anti-B2GP1) is associated with antiphospholipid syndrome (APS), a rare autoimmune disease that causes venous thromboembolism (VTE). APOH, which expresses the B2GP1 antigen, has been identified as a susceptible locus for positive anti-B2GP1 IgG. We investigated the impact of APOH locus polymorphisms on the relationship between anti-B2GP1 and VTE based on a genome-wide association study (GWAS).
Methods: We performed a GWAS on the total anti-B2GP1 levels as a quantitative trait in 5,830 adults enrolled in the Multi-Ethnic Study of Atherosclerosis (MESA), a multi-ancestral cohort sampled from the general population. Next, we obtained the summary statistics of VTE from a published GWAS (PMID: 36658437). For the APOH locus, we performed Mendelian randomization (MR) followed by colocalization with VTE as the outcome and total anti-B2GP1 as the exposure.
To identify the candidate causal SNP mediating the association between anti-B2GP1 and VTE, we obtained cis-protein quantitative trait locus (cis-pQTL) statistics on plasma B2GP1 levels from the UK Biobank and regulatory element predictions from the Roadmap Epigenomics Project. We performed Bayesian colocalization, statistical fine-mapping, functional annotations, and variant effect predictions.
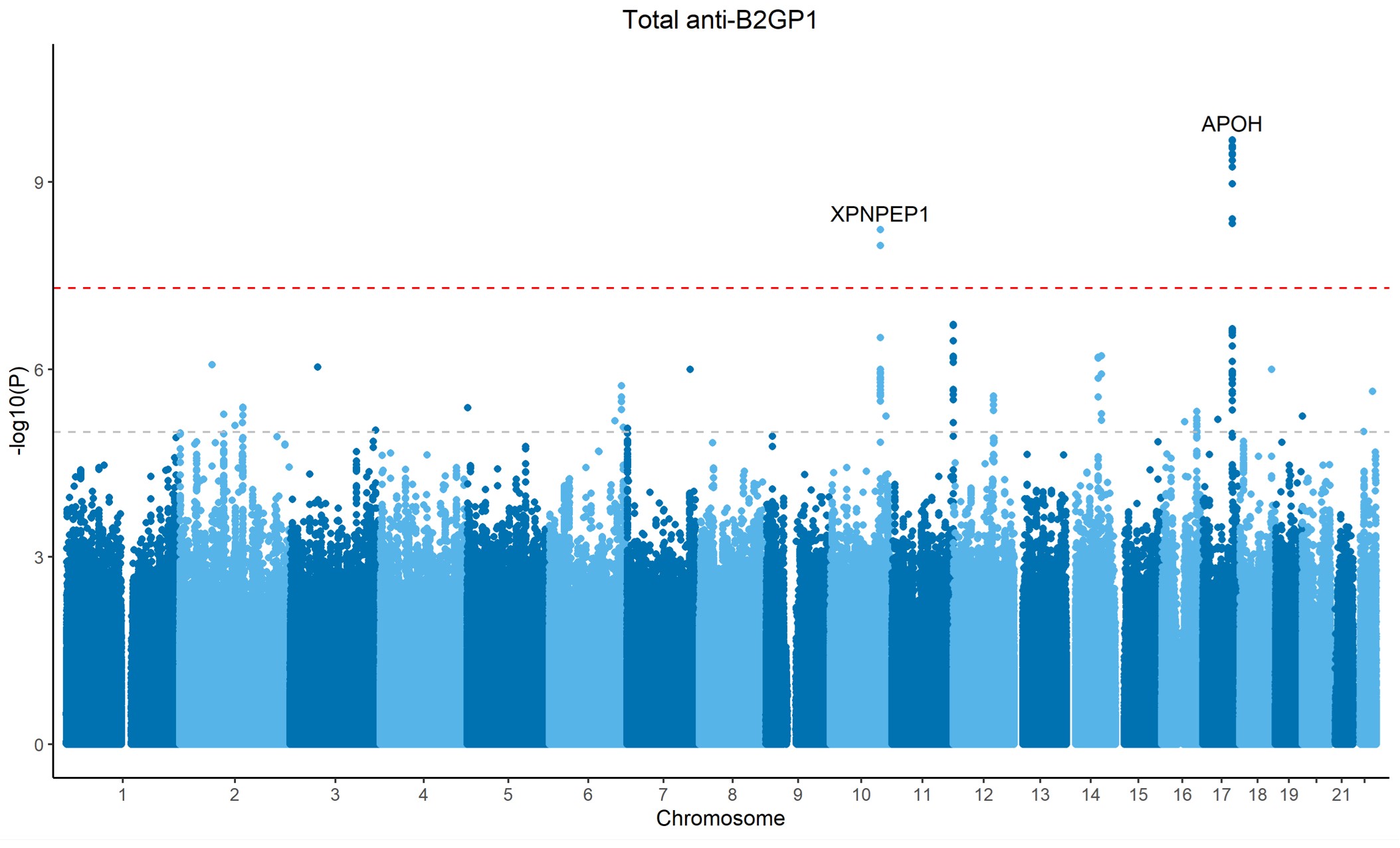
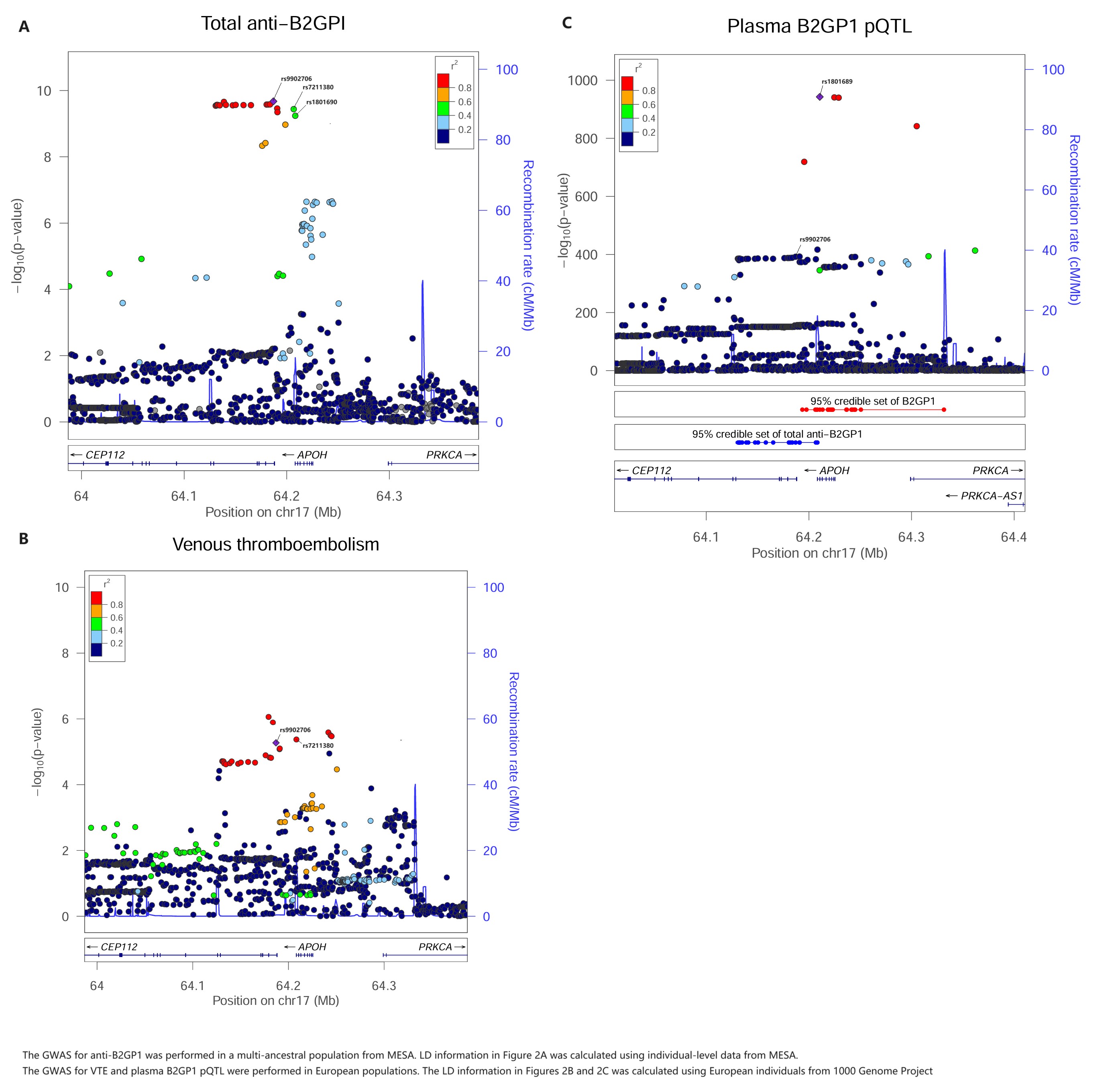
Results: APOH locus was significant for total anti-B2GP1 (p = 2.1 x 10-10, Figure 1). In this locus, genetically determined total anti-B2GP1 was associated with a reduced risk of VTE in MR (Beta = -0.20, p = 5.2 x 10-6), and a shared causal variant was predicted in the colocalization (posterior probability of colocalization [PP4] = 99.8%) (Figure 2A and 2B).
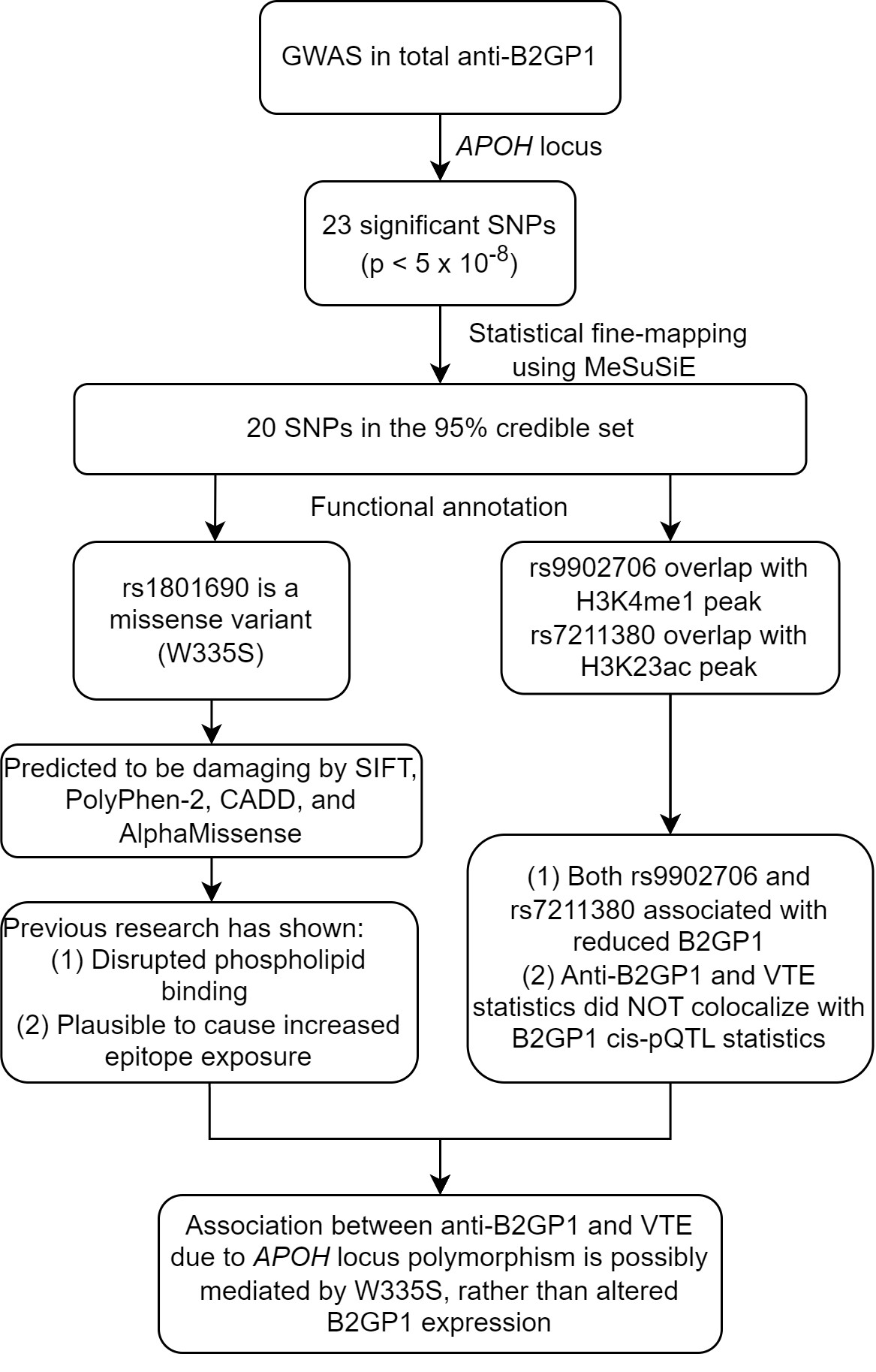
Statistical fine-mapping prioritized a 95% credible set of 20 SNPs, among which one was a missense variant (rs1801690, W335S) and two (rs9902706 and rs7211380) were in predicted enhancer regions. However, both rs9902706 and rs7211380 were associated with reduced plasma B2GP1 protein levels from cis-pQTL, and neither the statistics for anti-B2GP1 nor VTE colocalized with the statistics of plasma B2GP1 cis-pQTL (PP4 = 0, Figure 2C). The missense variant W335S was predicted to have a damaging effect by multiple bioinformatic predictors, including SIFT, PolyPhen-2, CADD, and AlphaMissense (Figure 3).
Conclusion: Genetically determined total anti-B2GP1 levels due to APOH locus polymorphisms are associated with a paradoxically reduced risk of VTE. We could not find convincing evidence that this association was mediated by altered B2GP1 expression. Instead, we identified a missense variant in B2GP1, W335S, which is plausible to mediate this association. The W335S variant is located in Domain V and has been found to disrupt B2GP1 binding to phospholipids, a critical step for anti-B2GP1-mediated thrombosis. Additionally, it has been established that certain disruptions in Domain V can cause a global structural change in B2GP1, leading to increased epitope exposure. Further research is warranted to investigate whether W335S has a similar effect, resulting in higher levels of anti-B2GP1.
To cite this abstract in AMA style:
Luo Y, Liu L, Khan A, Barr R, Bernstein E, Kiryluk K. APOH Locus Associated with Higher Anti-Beta-2-Glycoprotein 1 Antibody Levels Paradoxically Protects Against Venous Thromboembolism [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/apoh-locus-associated-with-higher-anti-beta-2-glycoprotein-1-antibody-levels-paradoxically-protects-against-venous-thromboembolism/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/apoh-locus-associated-with-higher-anti-beta-2-glycoprotein-1-antibody-levels-paradoxically-protects-against-venous-thromboembolism/