Session Information
Date: Friday, November 6, 2020
Title: Epidemiology & Public Health Poster I: COVID-19 & Rheumatic Disease
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Antirheumatic disease therapies have been used to treat coronavirus disease 2019 (COVID-19) and its complications. There has been particular interest in the antimalarial agent hydroxychloroquine (HCQ) and in agents that inhibit interleukin-1 (IL1) or interleukin-6 (IL-6). We conducted a systematic review and meta-analysis to describe the current evidence.
Methods: A search of published and preprint databases in all languages was performed on 3/19/2020 and updated on 05/07/2020. Included studies described one or more relevant clinical outcomes in five or more people who were infected with SARS-CoV-2 and were treated with antirheumatic disease therapy. Pairs of reviewers screened articles and extracted data. The risk of bias was assessed using the Newcastle-Ottawa Scale for cohort studies and the Risk of Bias 2.0 tool for randomized controlled trials (RCTs). A meta-analysis of effect sizes using random-effects models was performed when possible.
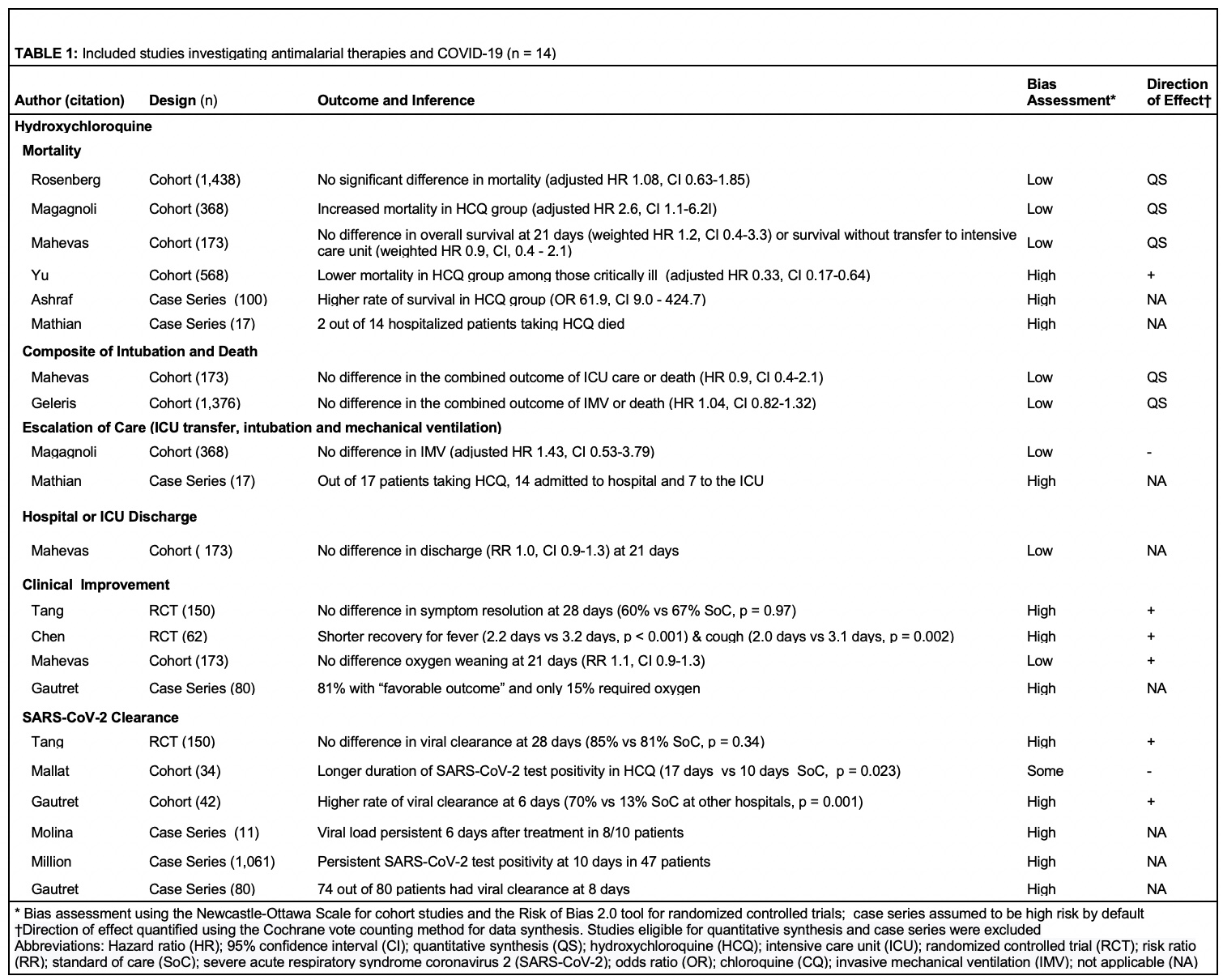
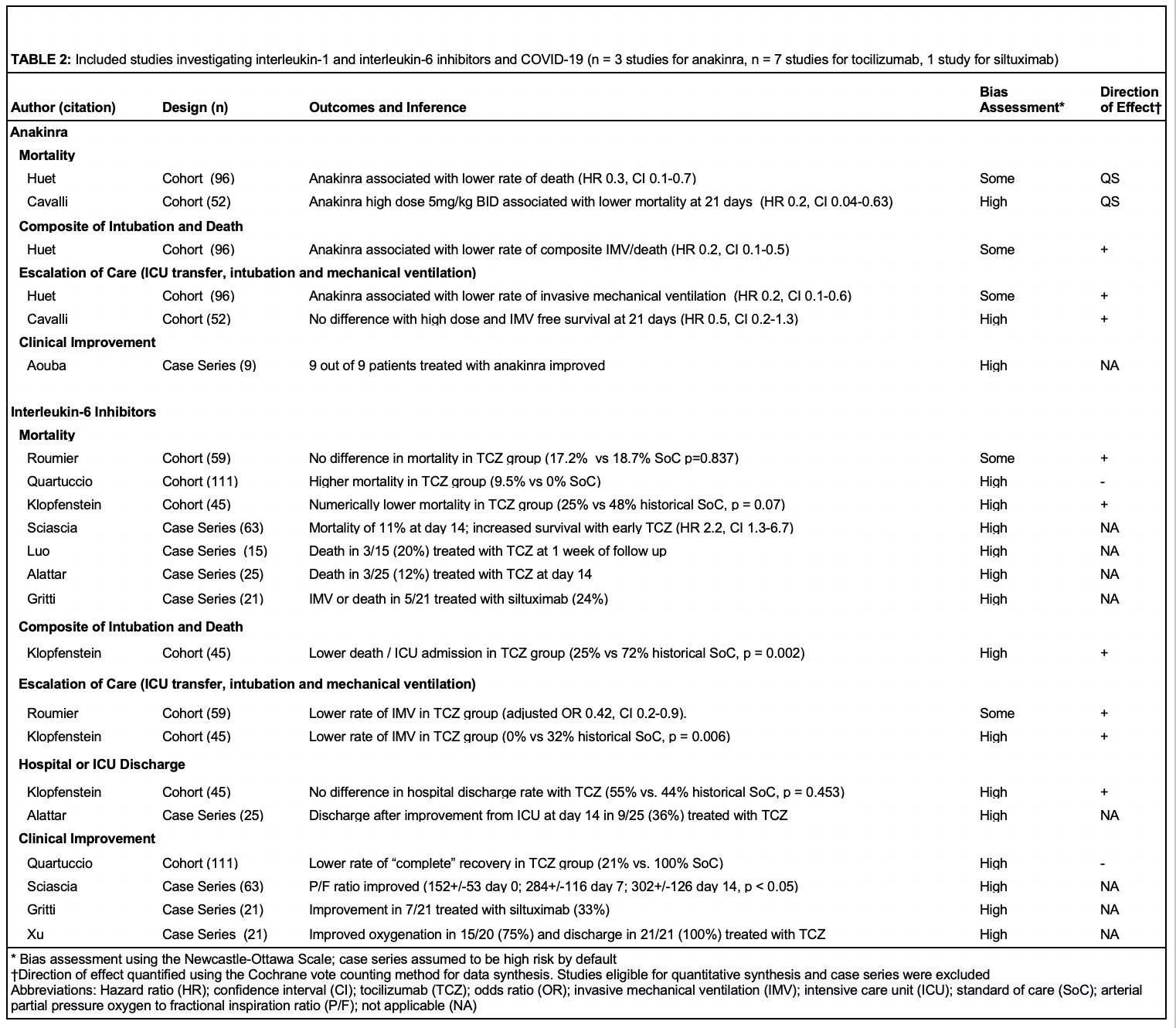
Results: The searches identified 3,935 articles, of which 275 were included for full text review. After full text exclusion, 45 studies were included in qualitative review (4 randomized controlled trials, 29 cohort studies, and 12 case series) and six studies were included in meta-analyses (4 cohort studies of HCQ, 2 cohort studies of anakinra). All studies evaluated hospitalized patients and 29 out of 45 had been published in a peer-reviewed journal. In a meta-analysis of three cohort studies with a low risk of bias, hydroxychloroquine use was not significantly associated with mortality (pooled hazard ratio (HR) 1.41, 95% confidence interval (CI) 0.83-2.42) (Figure 1A, Figure 1B, Table 1). In a meta-analysis of two cohort studies with some/high risk of bias, anakinra use was associated with lower mortality (pooled HR 0.2, 95% CI 0.1-0.4) (Figure 1C). A meta-analysis could not be performed on interleukin-6 inhibitor studies, which were frequently conflicting and had some/high risk of bias (Table 2). Studies that assessed glucocorticoids, intravenous immunoglobulin, and baricitinib also had conflicting results and the majority had a high risk of bias.
Conclusion: In this systematic review and meta-analysis, hydroxychloroquine use was not associated with benefit or harm with regard to COVID-19 mortality. The IL-1 inhibitor anakinra was associated with lower mortality, but this should be interpreted with caution given substantial risks of bias. Evidence supporting the effect of other antirheumatic disease therapies in COVID-19 is currently inconclusive, though randomized controlled trials are ongoing.
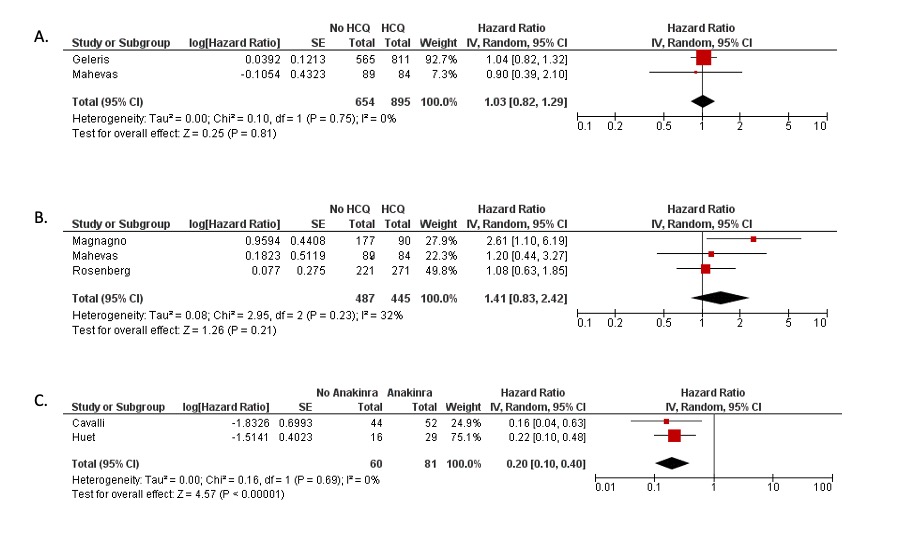 Figure 1A: Meta-analysis of two observational studies investigating hydroxychloroquine and the composite outcome of death or intubation among patients hospitalized with COVID-19 Figure 1B: Meta-analysis of three observational studies investigating hydroxychloroquine and mortality among patients hospitalized with COVID-19 Figure 1C: Meta-analysis of two observational studies investigating anakinra and mortality among patients hospitalized with COVID-19.
Figure 1A: Meta-analysis of two observational studies investigating hydroxychloroquine and the composite outcome of death or intubation among patients hospitalized with COVID-19 Figure 1B: Meta-analysis of three observational studies investigating hydroxychloroquine and mortality among patients hospitalized with COVID-19 Figure 1C: Meta-analysis of two observational studies investigating anakinra and mortality among patients hospitalized with COVID-19.
To cite this abstract in AMA style:
Putman M, Chock Y, Tam H, Kim A, Sattui S, Berenbaum F, Danila M, Korsten P, Sanchez Alvarez C, Sparks J, Coates L, Palmerlee C, Pierce A, Jayatilleke A, Johnson S, Kilian A, Liew J, Prokop L, Murad H, Grainger R, Wallace Z, Duarte-Garcia A. Antirheumatic Disease Therapies in Patients with COVID-19: A Systematic Review and Meta-analysis [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/antirheumatic-disease-therapies-in-patients-with-covid-19-a-systematic-review-and-meta-analysis/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/antirheumatic-disease-therapies-in-patients-with-covid-19-a-systematic-review-and-meta-analysis/