Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: The utility of measuring serial antineutrophil cytoplasmic autoantibody (ANCA) levels to guide treatment in granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA) is controversial. This analysis reports on circulating myeloperoxidase (MPO) and proteinase-3 (PR3) ANCA levels in patients in the avacopan and prednisone taper arms of the phase 3 ADVOCATE trial.
Methods: Serum MPO- and PR3-ANCA levels were measured by a central laboratory using an enzyme-linked immunosorbent assay for the following timepoints: day 1 (pre-treatment), week 13, 26, 39, and 52. Linear mixed effect models were used to evaluate the association of a) study treatment (avacopan or a prednisone taper); b) background therapy (rituximab or cyclophosphamide); and c) treatment response (sustained remission at week 52, yes or no) on MPO- or PR3-ANCA levels.
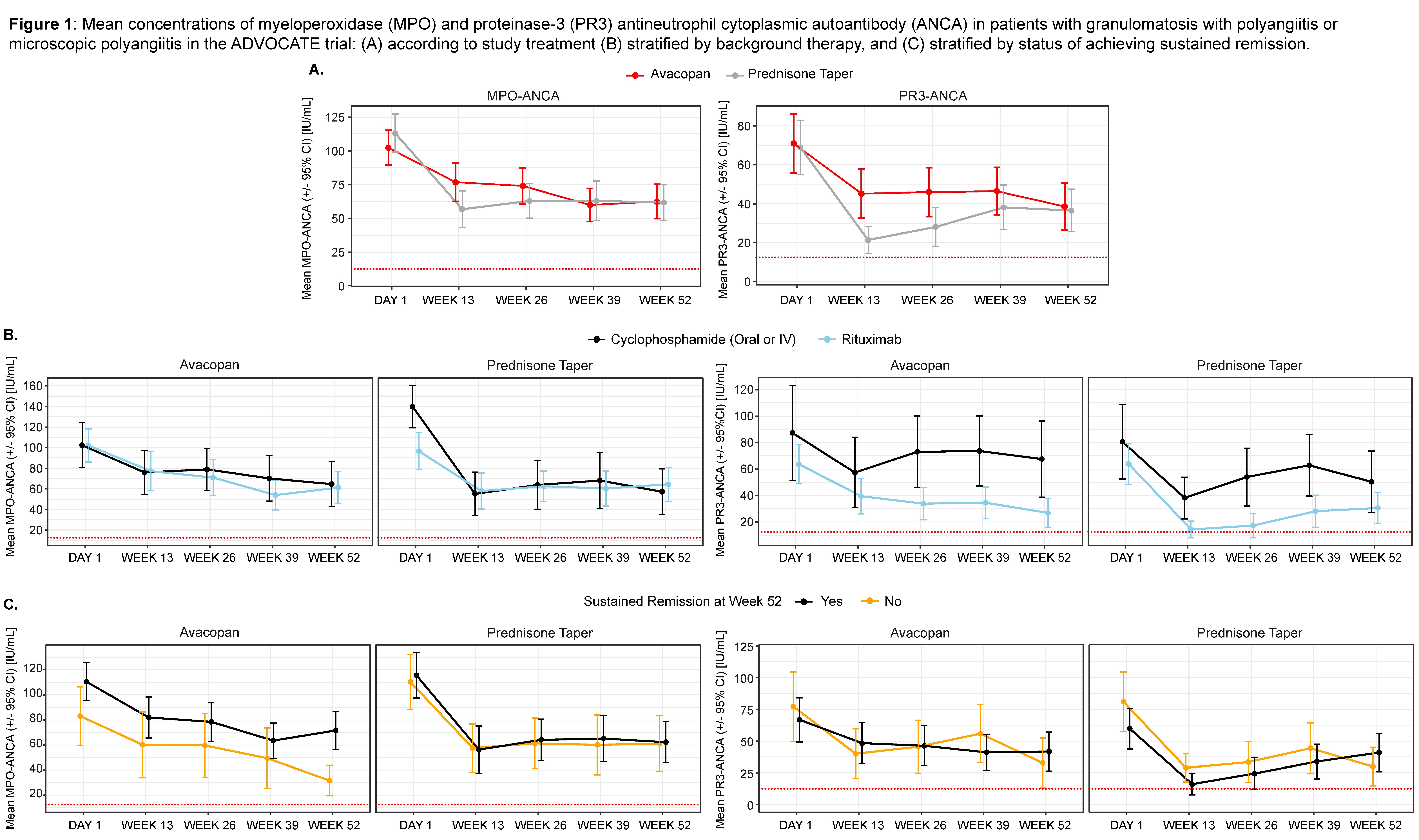
Results: In patients with MPO-ANCA (n=142) or PR3-ANCA (n=188), baseline mean MPO-ANCA levels were higher than PR3-ANCA levels, but both decreased from baseline over time. At week 13, mean ANCA levels decreased more in patients in the prednisone taper arm vs the avacopan arm (both PR3/MPO p=0.002), but levels converged by week 26 for MPO-ANCA and week 39 for PR3-ANCA (Figure 1A). While the decrease in mean PR3-ANCA was greater in patients treated with rituximab vs cyclophosphamide regardless of study treatment (p< 0.001), the mean MPO-ANCA trajectories were similar between background therapies (p=0.49) (Figure 1B). When stratified by treatment response, the reduction in mean ANCA levels was independent of sustained remission status (Figure 1C).
Conclusion: The addition of avacopan to rituximab or cyclophosphamide for treatment of GPA or MPA does not appear to impact ANCA levels. The lack of correlation between ANCA levels and outcome of treatment in the ADVOCATE trial suggests that ANCA levels are an unreliable marker of treatment response.
To cite this abstract in AMA style:
Cortazar F, Geetha D, Almaani S, Song C, Wilmanski T, Bozeman A, Merkel P, Jayne D. Antineutrophil Cytoplasmic Autoantibody Levels in Patients in the Avacopan Phase 3 Trial [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/antineutrophil-cytoplasmic-autoantibody-levels-in-patients-in-the-avacopan-phase-3-trial/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/antineutrophil-cytoplasmic-autoantibody-levels-in-patients-in-the-avacopan-phase-3-trial/