Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: Porphyromonas gingivalis (Pg) has received considerable attention by investigators trying to explain the striking association of periodontal disease (PD) and rheumatoid arthritis (RA). Bacterial Pg peptidylarginine deiminase (PPAD) has provided an exciting model to mechanistically link bacterial-induced citrullination and loss of tolerance to citrullinated proteins observed in RA. The recent finding that autocitrullinated GST-His-tagged PPAD is preferentially recognized by antibodies in RA has focused interest on PPAD as a bacterial target of anti-citrullinated protein antibody (ACPA) initiation. This observation would have significant implications on our understanding of RA pathogenesis.
Methods: PPAD was cloned from Pg strain W83 (ATCC) into pET-28a(+). Mutant PPADC351S lacking the capacity to autocitrullinate was generated by site-directed mutagenesis. PPAD and PPADC351S containing only His-tags were purified by affinity chromatography. Patients with RA (n=77) and healthy controls (n=39) were assayed for IgG antibodies to autocitrullinated PPAD (citPPAD) and PPADC351S by ELISA. To minimize error, antibodies to both citPPAD and PPADC351S were assayed on the same plate. Arbitrary units were corrected for background from individual sera given by PBS-coated wells. Radiolabeled PPAD generated by in vitro transcription/translation was immunoprecipitated by RA sera. Antibodies to cyclic citrullinated peptides (CCP) were assayed by QUANTA Lite CCP3 IgG ELISA (INOVA). Correlation between anti-CCP3 units and anti-PPAD levels was analyzed using Spearman’s correlation. Statistical analysis of PPAD ELISA groups was performed using the Wilcoxon and Mann-Whitney test, when appropriate.
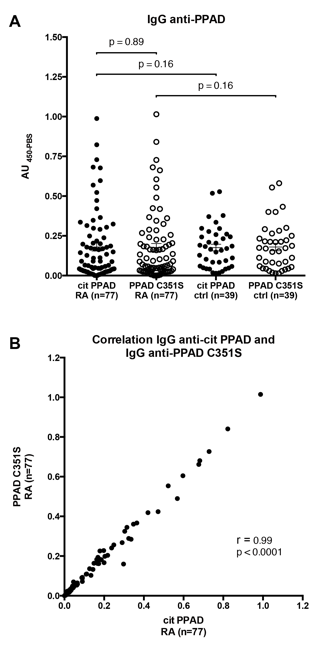
Results: PPAD, but not PPADC351S, demonstrated prominent autocitrullination when expressed in E. coli BL21(DE3). Antibodies to citPPAD and PPADC351S did not differ significantly among patients with RA (p=0.89; Fig. 1A). Correlation of citPPAD and PPADC351S antibody levels was highly significant (r=0.99, p<0.0001; Fig. 1B). Anti- citPPAD and anti-PPADC351S levels were not significantly different in RA compared to controls (p=0.16). Anti-CCP3 antibodies were found in 74% of RA patients (57/77), and levels were not correlated with anti-citPPAD or anti-PPADC351S (r=0.04, p=ns; r=0.07, p=ns). Sera from RA patients with high titer anti-CCP3 did not immunoprecipitate radiolabeled PPAD.
Conclusion: Anti-PPAD antibodies are present in RA, but demonstrate no preferential reactivity to autocitrullinated PPAD. Levels of anti-PPAD do not differ significantly between RA and controls. Anti-CCP antibodies do not correlate with anti-PPAD levels in RA, and do not cross-react with PPAD. Our findings suggest that PPAD autocitrullination is not the underlying mechanism linking Pg-associated PD and RA.
Fig. 1. Antibodies to citPPAD and PPADC351S in RA and healthy controls.
Disclosure:
M. F. Konig,
None;
A. Paracha,
None;
M. Moni,
None;
C. O. Bingham III,
None;
F. Andrade,
None.
« Back to 2013 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/antibodies-to-porphyromonas-gingivalis-peptidylarginine-deiminase-in-rheumatoid-arthritis-are-not-directed-against-the-autocitrullinated-enzyme/