Session Information
Date: Sunday, November 17, 2024
Title: Innate Immunity Poster
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: A growing body of evidence suggests that VISTA, an immune checkpoint inhibitory receptor, plays a central role in the regulation of innate immunity in the settings of inflammatory diseases. Neutrophils are among the cells that have the highest membrane density of surface VISTA. Here we test if antibody targeting of VISTA can suppress neutrophil accumulation and ameliorate systemic and tissue inflammation.
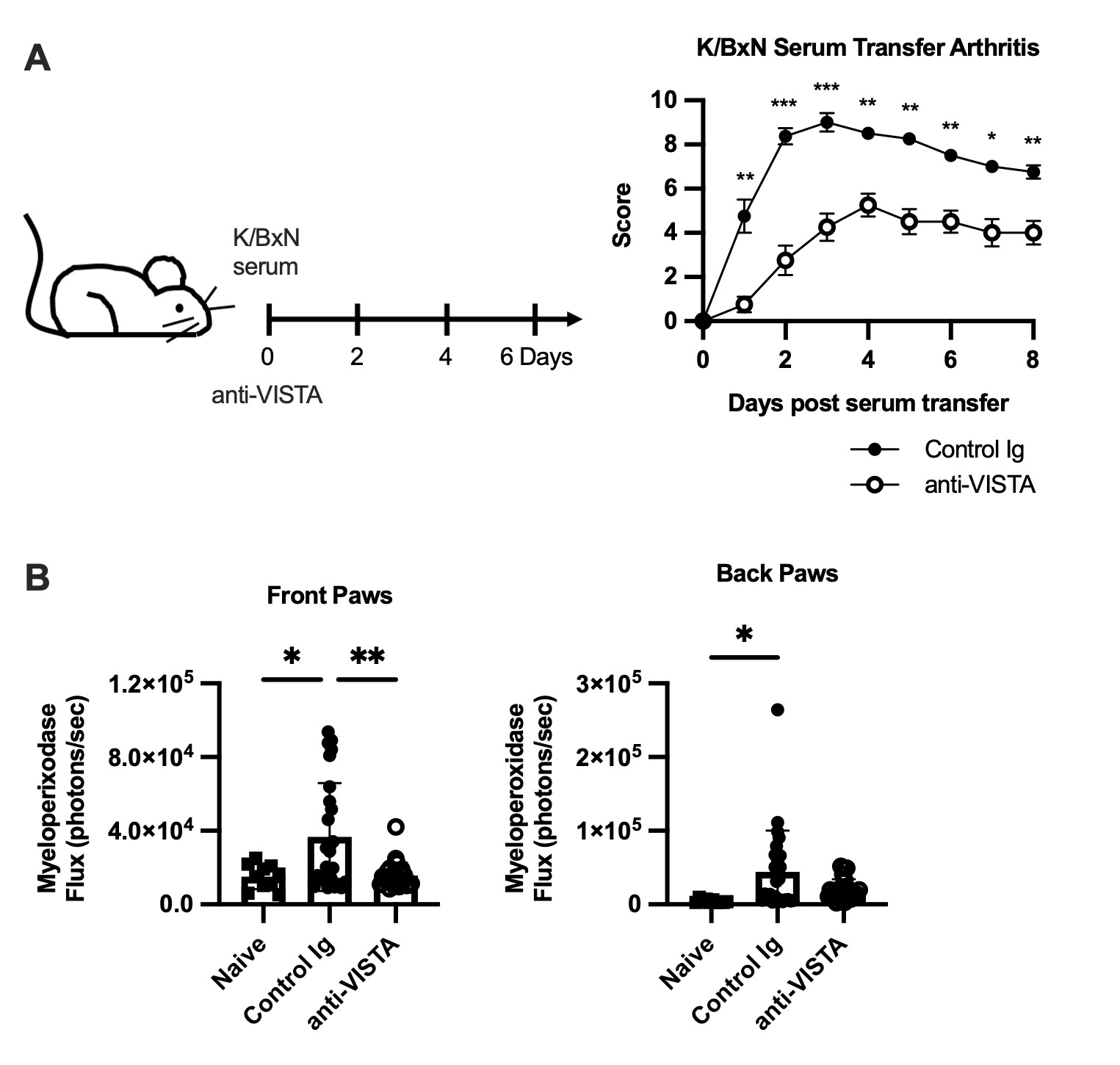
Methods: B6, human VISTA knock-in (hVISTAKI), S100a8creVsirfl/fl (deficient in VISTA only in neutrophils), Fcer1g-/-, and Fcgr2b -/- mice were all treated i.p. with LPS (10 mg), CXCL2 (1 mg) or vehicle and anti-VISTA or control antibodies (8G8 and 803 mAb clones, 200 mg). Neutrophil levels and viability were quantified in the bone marrow, spleen, lung, liver 12 and 20hr after antibody treatment by flow cytometry. Mice were injected with K/BxN serum on day 0 and anti-VISTA or control antibody on days 0, 2, 4, and 6. Disease severity and MPO flux were quantified.
Results: Treatment with anti-VISTA antibody suppresses LPS-induced neutrophil accumulation in the spleen and blood, in both B6 (8G8) and hVISTAKI (803) mice, without suppressing neutrophil egress from the bone marrow. VISTA expression on neutrophils is required as no reduction in the peripheral or bone marrow neutrophils was observed in S100a8creVsirfl/flmice after LPS, compared to cre-Vsirfl/fl controls. Fcer1g-/- mice treated with anti-VISTA mAb showed no decrease in neutrophil numbers in the spleen after LPS challenge. In contrast, anti-VISTA mAb potently suppressed splenic neutrophil accumulation in the Fcgr2b-/- mice. Transfer experiments showed that the FcR on other cells, but not neutrophils, is required for the anti-VISTA mAb effects. The frequency of SYTOX+ neutrophils was increased 2-fold in the liver with anti-VISTA mAb after LPS challenge and neutrophil extracellular traps were confirmed by immunofluorescence staining of the liver. Similar to the findings with LPS, splenic neutrophil numbers were reduced in the anti-VISTA mAb treated group after CXCL2 treatment, which was paralleled by an increase in the percent of SYTOX+ neutrophils. In the K/BxN serum transfer neutrophil-mediated model of arthritis, anti-VISTA mAb treatment markedly reduced disease severity, which was associated with reduced myeloperoxidase activity in the joints (Figure 1).
Conclusion: These studies identify a novel therapeutic opportunity for targeting VISTA to control neutrophil-mediated inflammation and tissue injury by promoting neutrophil clearance in the liver, potentially via NETosis. Of relevance to arthritis, our data suggest that anti-VISTA mAb reduces disease severity by inhibiting neutrophil accumulation in the joint.
To cite this abstract in AMA style:
Nowak E, Li J, ElTanbouly M, Mendyka L, Lines L, Mabaera R, Burns C, Noelle R, Skopelja-Gardner S. Anti-VISTA Antibody Suppresses Neutrophil-mediated Inflammation [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/anti-vista-antibody-suppresses-neutrophil-mediated-inflammation/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/anti-vista-antibody-suppresses-neutrophil-mediated-inflammation/