Session Information
Date: Tuesday, October 28, 2025
Title: (2377–2436) Systemic Lupus Erythematosus – Diagnosis, Manifestations, & Outcomes Poster III
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Systemic Lupus Erythematosus (SLE) is a chronic autoimmune disease that causes inflammation in many of the body’s tissues, including the heart. Recent studies attribute almost 50% of mortality in lupus patients to cardiovascular (CV) disease after the first 10 years. Myocardial inflammation is present in up to 40% of lupus patients. Understanding the pathogenic mechanisms that drive CV disease in SLE is essential for its management and for developing new therapeutic approaches. Given the importance of the membrane barrier function in preventing exposure of intracellular antigens to the extracellular space where they could act as autoantigens, compromised membrane repair may be a contributory mechanism to SLE pathogenesis. We identified TRIM72, which is essential for membrane repair, as an autoantigen in myositis patients. Our current studies indicate this may be the case in lupus as well. Given the potential contribution of membrane repair in development of lupus myocarditis and the role of TRIM72 in membrane repair, we hypothesized that defective membrane repair leads to aberrant extracellular exposure of membrane repair proteins, such as TRIM72, and autoantibody generation which creates a positive feedback loop causing further exposure of intracellular antigens contributing to lupus myocarditis pathogenesis.
Methods: A custom TRIM72 antibody ELISA was used to quantify serum levels of TRIM72 antibodies in serum. Multi-photon confocal laser microscopy was used to measure the dynamics of membrane repair in vitro. snRNAseq of NZM2410 mouse hearts was performed on young and old mice. Partek™ Flow™ software was used to analyze the transcriptomic expression data.
Results: We demonstrate that anti-TRIM72 auto-antibodies were elevated in both SLE patient serum diagnosed with myocarditis and serum of NZM2410 mice with myocarditis. Murine cardiomyocyte membrane repair is significantly reduced by exposure to myocarditis positive SLE patient serum with high levels of TRIM72 antibodies but not to healthy control serum. Removal of TRIM72 antibodies from serum reduces this effect on membrane repair. Preliminary data demonstrate similar trends of lupus sera containing anti-TRIM72 antibodies on human cardiomyocytes. snRNAseq revealed that cardiomyocytes are reduced and fibroblasts increase as NZM2410 mice age. Specific CV disease pathways are enriched in cells of aged hearts of NZM2410 mice as compared to young mice. Genes linked to lupus disease pathology are differentially expressed in immune cells. Subcluster analysis of specific cell populations, e.g. cardiomyocytes, fibroblasts, etc. revealed several cell types and gene expression profiles associated with development of SLE myocarditis.
Conclusion: The plasma membrane repair protein TRIM72 is an autoantigen in SLE associated myocarditis and potentially contributes to disease pathogenesis. We demonstrate the presence of TRIM72 antibodies in SLE patients that can compromise membrane repair in cardiomyocytes in vitro. Ongoing studies will examine if these defects lead to membrane repair protein exposure to the extracellular space causing the production of autoantibodies which contribute to a vicious cycle that exacerbates SLE pathology.
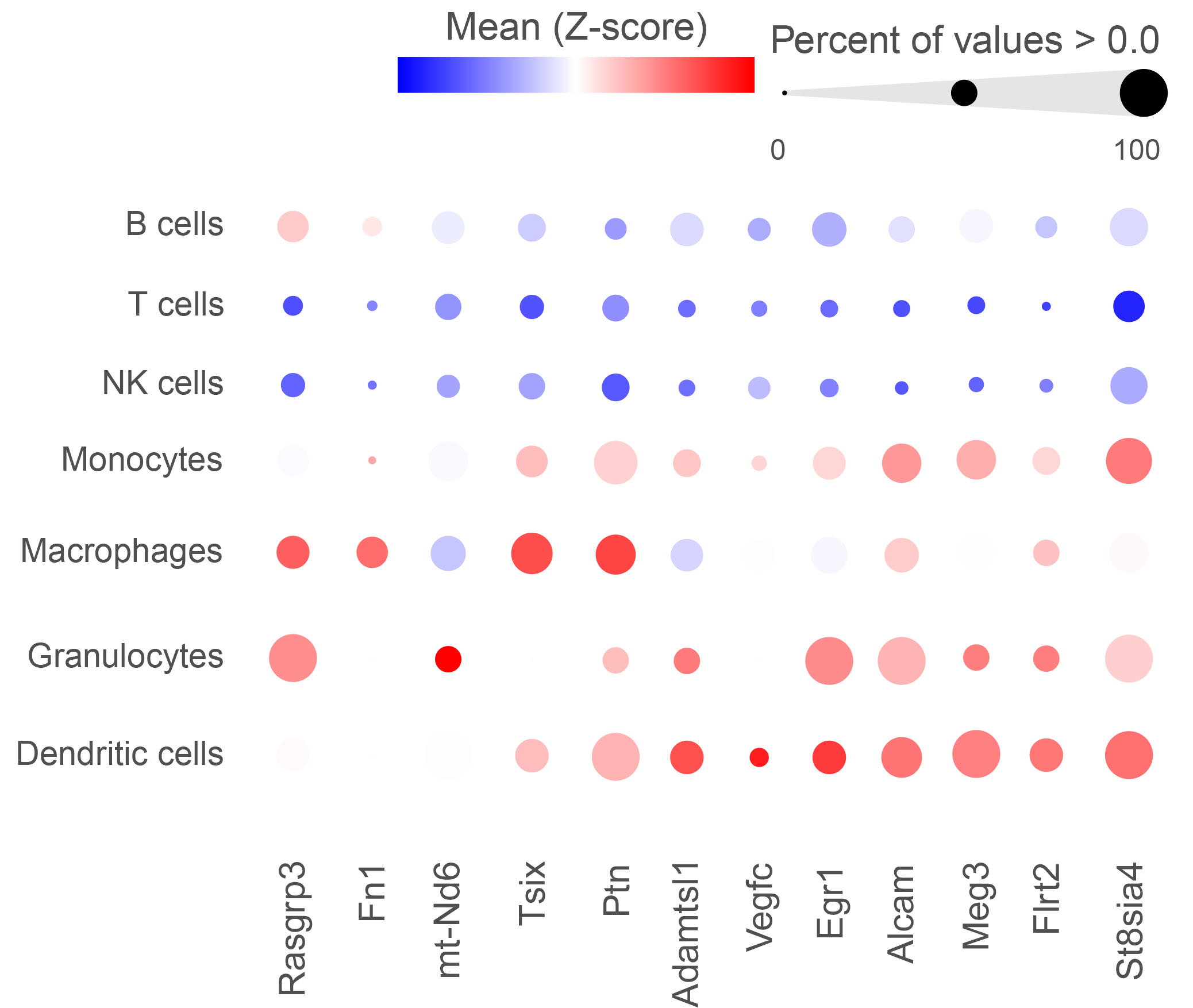 Transcriptomic analysis of cardiac tissue reveals multiple differentially expressed genes linked to lupus. A partial list of statistically significant differentially expressed genes in immune cells is shown.
Transcriptomic analysis of cardiac tissue reveals multiple differentially expressed genes linked to lupus. A partial list of statistically significant differentially expressed genes in immune cells is shown.
To cite this abstract in AMA style:
Zeno B, Bruckner S, Banford k, Bulgart H, Ardoin S, weisleder n, Jarjour W. Anti-Trim72 Auto-antibodies In Systemic Lupus Erythematosus Patients and a Lupus Mouse Model with Myocarditis Compromise Membrane Repair in Mouse & Human Cardiomyocytes [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/anti-trim72-auto-antibodies-in-systemic-lupus-erythematosus-patients-and-a-lupus-mouse-model-with-myocarditis-compromise-membrane-repair-in-mouse-human-cardiomyocytes/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/anti-trim72-auto-antibodies-in-systemic-lupus-erythematosus-patients-and-a-lupus-mouse-model-with-myocarditis-compromise-membrane-repair-in-mouse-human-cardiomyocytes/
