Session Information
Session Type: Poster Session B
Session Time: 8:30AM-10:30AM
Background/Purpose: To longitudinally follow the levels of anti- transcriptional intermediary factor (TIF)1-gamma autoantibodies in patients with dermatomyositis with and without cancer.
Methods: We identified sera from 128 patients diagnosed with dermatomyositis (DM) between 1996 and May 2018 from a national myositis cohort. DM diagnosis was based on the new 2017 Eular/ACR classification criteria for inflammatory myopathies. Serum samples taken at DM diagnosis and after one and three years were tested for anti-TIF1-gamma autoantibodies by Enzyme-Linked ImmunoSorbent Assay (ELISA). Clinical data on DM and cancer was extracted from medical records, National Rheumatology Quality Register, National Myositis Network, International Myositis Register and National Cancer Register. Patients were grouped based on cancer within +/-3 years and anti-TIF1-gamma status into four mutually exclusive groups: DM negative or positive for anti-TIF1-gamma autoantibody without cancer +/-3 years (DM- and DM+) and DM with cancer +/-3 years (CADM) negative or positive for anti-TIF1-gamma autoantibody (CADM- and CADM+). Levels of antibody over time were compared with healthy controls and between subgroups.
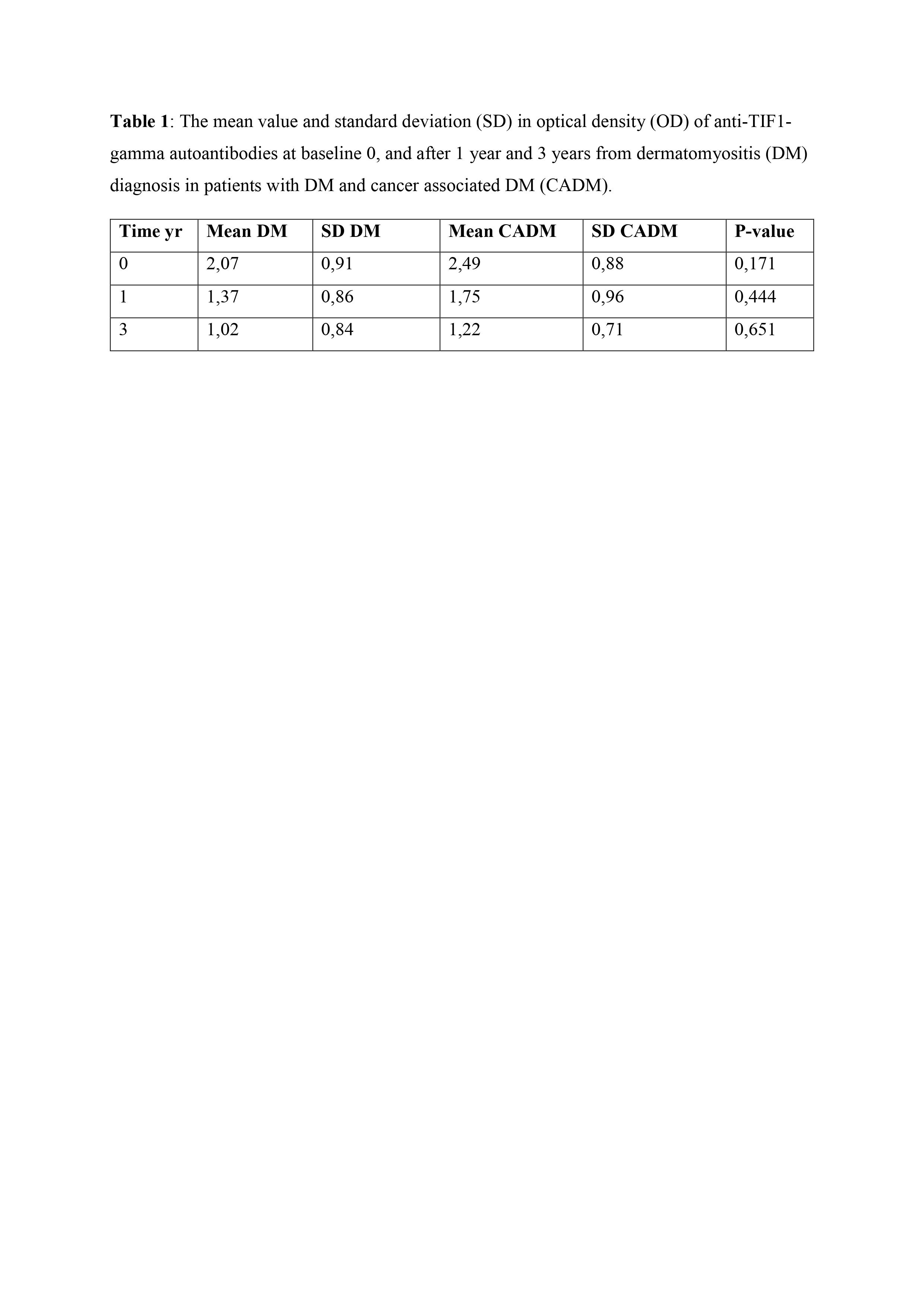
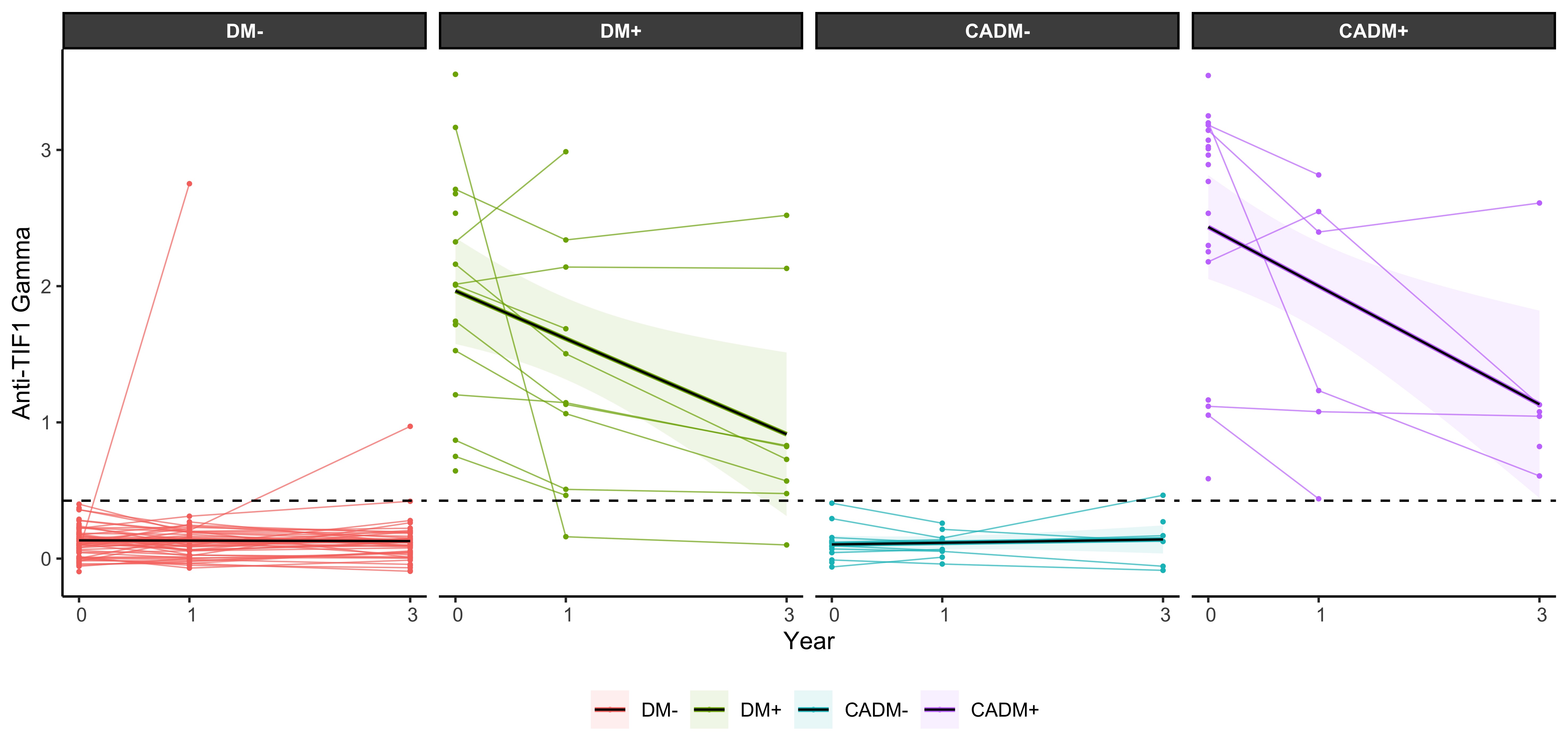
Results: For 41 patients only one serum sample was available, while 87 had longitudinal samples: 33 patients had 2 serum samples and 54 patients had 3 serum samples. Of all 128 patients, 34 (27%) had cancer within 3 years from myositis diagnosis and 36 (28%) of 128 were positive for anti-TIF1-gamma autoantibodies. We found 78 DM- (61%), 17 DM+ (13%), 19 CADM+ (15%) and 15 CADM- (12%). The mean serum level of anti-TIF1-gamma autoantibodies at baseline and after one and three years was higher in CADM+ patients compared to DM+ (table 1 and figure 1). Patients who tested positive at diagnosis for anti-TIF1-gamma autoantibodies, regardless of cancer status, lightly decreased in serum levels over time but remained in general persistently positive. During the observation time, only two patients changed their anti-TIF1-gamma autoantibody status from negative to positive and one from positive to negative.
Conclusion: The variation of anti-TIF1-gamma autoantibody levels over time did not differ in patients with DM with or without cancer and change in antibody status is rare, indicating that repeated serological tests might not be helpful in the clinical practice.
To cite this abstract in AMA style:
Dani L, Selickaja S, Galindo-Feria A, Svensson J, Venalis P, Leonard D, Hemberg M, Hanna B, Lundberg I, Holmqvist M. Anti-Transcriptional Intermediary Factor 1-gamma Antibodies in Dermatomyositis with and Without Cancer – A Longitudinal Study [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/anti-transcriptional-intermediary-factor-1-gamma-antibodies-in-dermatomyositis-with-and-without-cancer-a-longitudinal-study/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/anti-transcriptional-intermediary-factor-1-gamma-antibodies-in-dermatomyositis-with-and-without-cancer-a-longitudinal-study/