Session Information
Date: Saturday, November 7, 2020
Title: RA – Diagnosis, Manifestations, & Outcomes Poster II: Biomarkers
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Over the past years, novel biomarkers have been identified in the sera of rheumatoid arthritis (RA) patients, including autoantibodies to the protein-arginine deiminase (PAD) enzymes. These proteins are involved in the disease pathogenesis, and a total of five PAD family members (PAD1, 2, 3, 4 and 6) have been described in humans, of which PAD2, PAD3 and PAD4, and more recently PAD1, have been identified as autoantigenic targets. Nevertheless, fewer studies have explored the clinical value of anti-PAD1 antibodies. Especially anti-PAD4 antibodies have been shown to help in the diagnosis of RA. The objective of this study was to evaluate the presence of anti-PAD1 and anti-PAD4 IgG in the sera of RA patients and to investigate the utility of an anti-PAD1/PAD4 composite score.
Methods: Serum samples from RA patients (n=262) and controls (n=270) were used to evaluate the discriminatory power of antibodies targeting the individual PAD enzymes. All samples included in this study were tested for anti-PAD1, PAD2, PAD3, PAD4, and PAD6 IgG using the novel particle-based multi-analyte technology (PMAT, research use only, Inova Diagnostics, San Diego, USA). Anti-citrullinated protein antibodies (ACPA) IgG was also measured in these patients by QUANTA Lite CCP3 enzyme-linked immunoassay (ELISA, Inova Diagnostics, San Diego, US).
Results: Autoantibodies against all five PAD proteins were detected. Interestingly, anti-PAD1 were preferentially observed in patients with RA resulting in good discrimination between RA and controls from receiver operating characteristic (ROC) analysis (Figure 1). The area under the curve (AUC) for anti-PAD1 was comparable to the AUC obtained for anti-PAD4 antibodies, in both the total (AUC= 0.687, 95% CI 0.642-0.732 and AUC=0.696, 95% CI 0.652-0.740, respectively), and the ACPA negative population (AUC= 0.580, 95% CI .500-0.660 and AUC=0.530, 95% CI 0.450-0.610, respectively). Combining anti-PAD1 and anti-PAD4 in a composite score improved the discrimination between RA and controls substantially (Table 1). In addition, anti-PAD1 and anti-PAD4 were found in the ACPA negative RA group where anti-PAD1 had slightly higher positivity than anti-PAD4.
Conclusion: Our study is one of the first to confirm PAD1 and PAD6 as autoantigenic targets in RA. Interestingly, anti-PAD1 antibodies were mostly found in patients with RA with high disease specificity and although a high degree of overlap with anti-PAD4 was observed, they were also observed in the absence of these anti-PAD antibodies. Anti-PAD1 antibodies hold promise to help to close the serological gap in RA and might be superior to anti-PAD4 in the ACPA negative population, but future studies are warranted to validate in larger populations. Moreover, the observation that anti-PAD1 and anti-PAD4 antibodies combined in a composite score increases the discrimination between RA and control has the potential to further improve the diagnosis of RA.
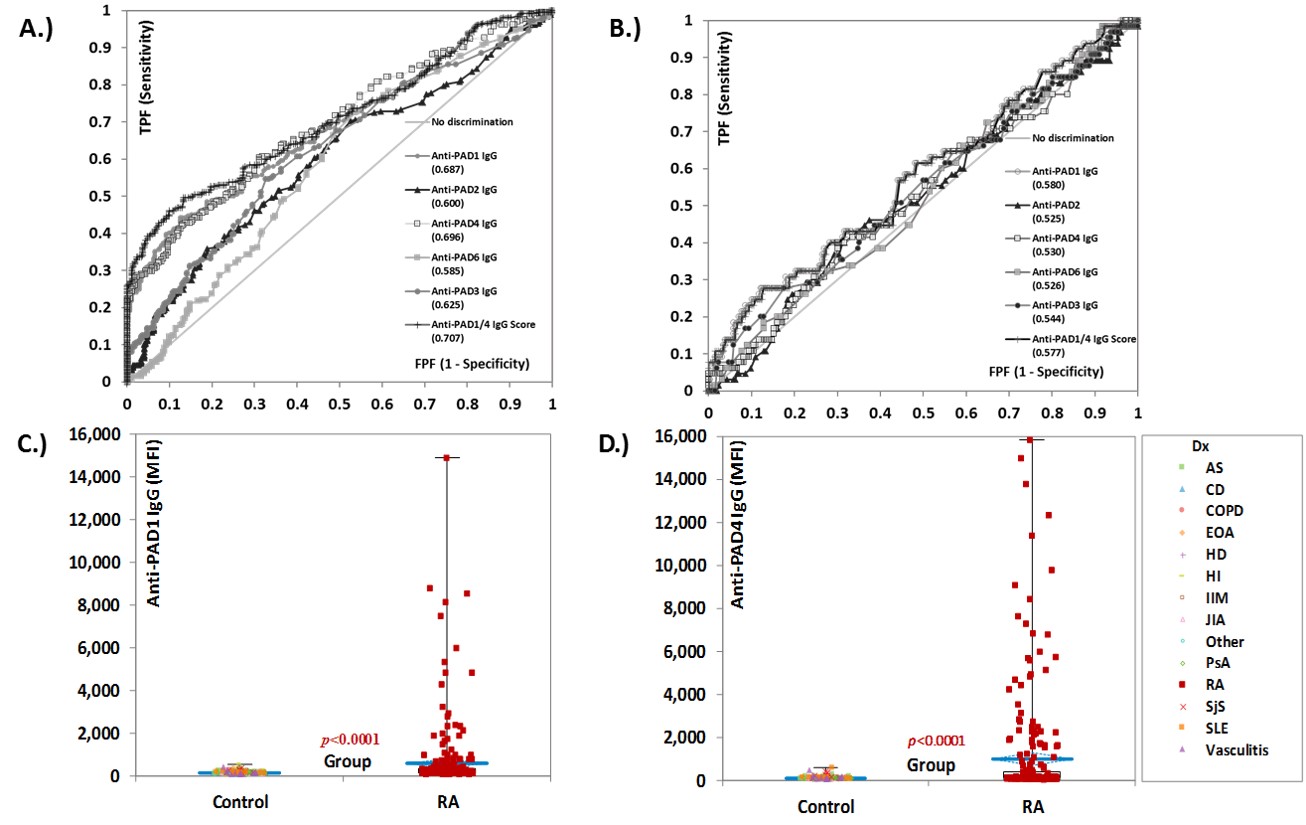 Figure 1 Diagnostic performance of individual antibodies to the protein-arginine deiminase (PAD) enzymes and of the anti-PAD1/4 IgG composite score. Receiver operating characteristic (ROC) analysis comparing the five individual anti-PAD antibodies and the anti-PAD1/4 composite score in their ability to discriminate between RA and controls in the total population (n=532) in panel A.), and in the ACPA negative individuals (n=321) in panel B.). Varying discrimination ability was observed, with anti-PAD1 and 4 showing the best diagnostic performance. The compositive PAD1/4 score shows improved discrimination between rheumatoid arthritis (RA) and controls. Area under the curve (AUC) values are shown for each of the anti-PAD antibodies. The pairwise comparison of the levels of anti-PAD1 and anti-PAD4 in patients with RA (n=262) vs controls (n= 270) are shown in C.). Panel D.) shows the same data for for anti-PAD4 IgG. Results are expressed in median fluorescence intensity (MFI). Abbreviations: AS: ankylosing spondylitis; CD: Chron’s disease; CI: confidence interval; COPD: chronic obstructive pulmonary disease; EOA: erosive osteoarthritis; FPF: false positive fraction; HD: Hashimoto’s disease; HI: healthy individuals; IIM: idiopathic inflammatory myopathies; JIA: juvenile idiopathic arthritis; MFI: median fluorescent intensity; PAD: protein-arginine deiminase; PsA: psoriatic arthritis; RA: Rheumatoid arthritis; SjS: Sjogren’s syndrome; SLE: systemic lupus erythematosus; TPF: true positive fraction.
Figure 1 Diagnostic performance of individual antibodies to the protein-arginine deiminase (PAD) enzymes and of the anti-PAD1/4 IgG composite score. Receiver operating characteristic (ROC) analysis comparing the five individual anti-PAD antibodies and the anti-PAD1/4 composite score in their ability to discriminate between RA and controls in the total population (n=532) in panel A.), and in the ACPA negative individuals (n=321) in panel B.). Varying discrimination ability was observed, with anti-PAD1 and 4 showing the best diagnostic performance. The compositive PAD1/4 score shows improved discrimination between rheumatoid arthritis (RA) and controls. Area under the curve (AUC) values are shown for each of the anti-PAD antibodies. The pairwise comparison of the levels of anti-PAD1 and anti-PAD4 in patients with RA (n=262) vs controls (n= 270) are shown in C.). Panel D.) shows the same data for for anti-PAD4 IgG. Results are expressed in median fluorescence intensity (MFI). Abbreviations: AS: ankylosing spondylitis; CD: Chron’s disease; CI: confidence interval; COPD: chronic obstructive pulmonary disease; EOA: erosive osteoarthritis; FPF: false positive fraction; HD: Hashimoto’s disease; HI: healthy individuals; IIM: idiopathic inflammatory myopathies; JIA: juvenile idiopathic arthritis; MFI: median fluorescent intensity; PAD: protein-arginine deiminase; PsA: psoriatic arthritis; RA: Rheumatoid arthritis; SjS: Sjogren’s syndrome; SLE: systemic lupus erythematosus; TPF: true positive fraction.
 Table 1 Diagnostic performance characteristics of anti-PAD1 and anti-PAD4 antibodies individually and in a composite score. Abbreviations: ACPA= anti-citrullinated protein antibodies; LR= likelihood ratio; OR= odds ratio; PAD= protein-arginine deiminase.
Table 1 Diagnostic performance characteristics of anti-PAD1 and anti-PAD4 antibodies individually and in a composite score. Abbreviations: ACPA= anti-citrullinated protein antibodies; LR= likelihood ratio; OR= odds ratio; PAD= protein-arginine deiminase.
To cite this abstract in AMA style:
Lopez-Hoyos M, Bentow C, Ishii A, Martinez-Prat L, Mahler M. Anti-Protein-Arginine Deiminase (PAD) 1 IgG Is a Promising Novel Autoantigen in Rheumatoid Arthritis (RA) and Increases Diagnostic Performance in Combination with Anti-PAD4 IgG Using a Composite Biomarker Score [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/anti-protein-arginine-deiminase-pad-1-igg-is-a-promising-novel-autoantigen-in-rheumatoid-arthritis-ra-and-increases-diagnostic-performance-in-combination-with-anti-pad4-igg-using-a-composite-bioma/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/anti-protein-arginine-deiminase-pad-1-igg-is-a-promising-novel-autoantigen-in-rheumatoid-arthritis-ra-and-increases-diagnostic-performance-in-combination-with-anti-pad4-igg-using-a-composite-bioma/
