Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Systemic sclerosis (SSc) is a complex autoimmune connective tissue disease that negatively impacts internal organ function, including the gastrointestinal tract. While the etiology of GI complications in patients with SSc remains unclear, some data demonstrate that anti-M3R antibodies may contribute to developing rapidly progressive intestinal pseudo-obstruction. We sought to (1) investigate the prevalence of anti-M3R antibodies in a cohort of patients with SSc and variable GI symptoms and (2) examine the GI and extra-intestinal manifestations associated with these antibodies.
Methods: Sera from 132 patients who met SSc criteria and had variable GI symptoms were tested for anti-M3R antibodies by second-generation enzyme-linked immunosorbent assay (ELISA). Positive antibody status was based on standard assay cutoffs. High-titer antibodies were defined by the highest-third of anti-M3R density in our cohort. Our cohort was enriched for patients with clinical GI manifestations (severe GI disease present in 17% of the cohort). Significant GI disease was defined by a Medsger GI severity score of 2-4 (i.e., antibiotics for bacterial overgrowth, the presence of pseudo-obstruction, or the requirement for total parenteral nutrition). Chi-squared and regression analyses were utilized to examine the associations between autoantibody status and titers, significant GI disease, and SSc clinical features captured over time at a tertiary referral center with a longitudinal patient registry.
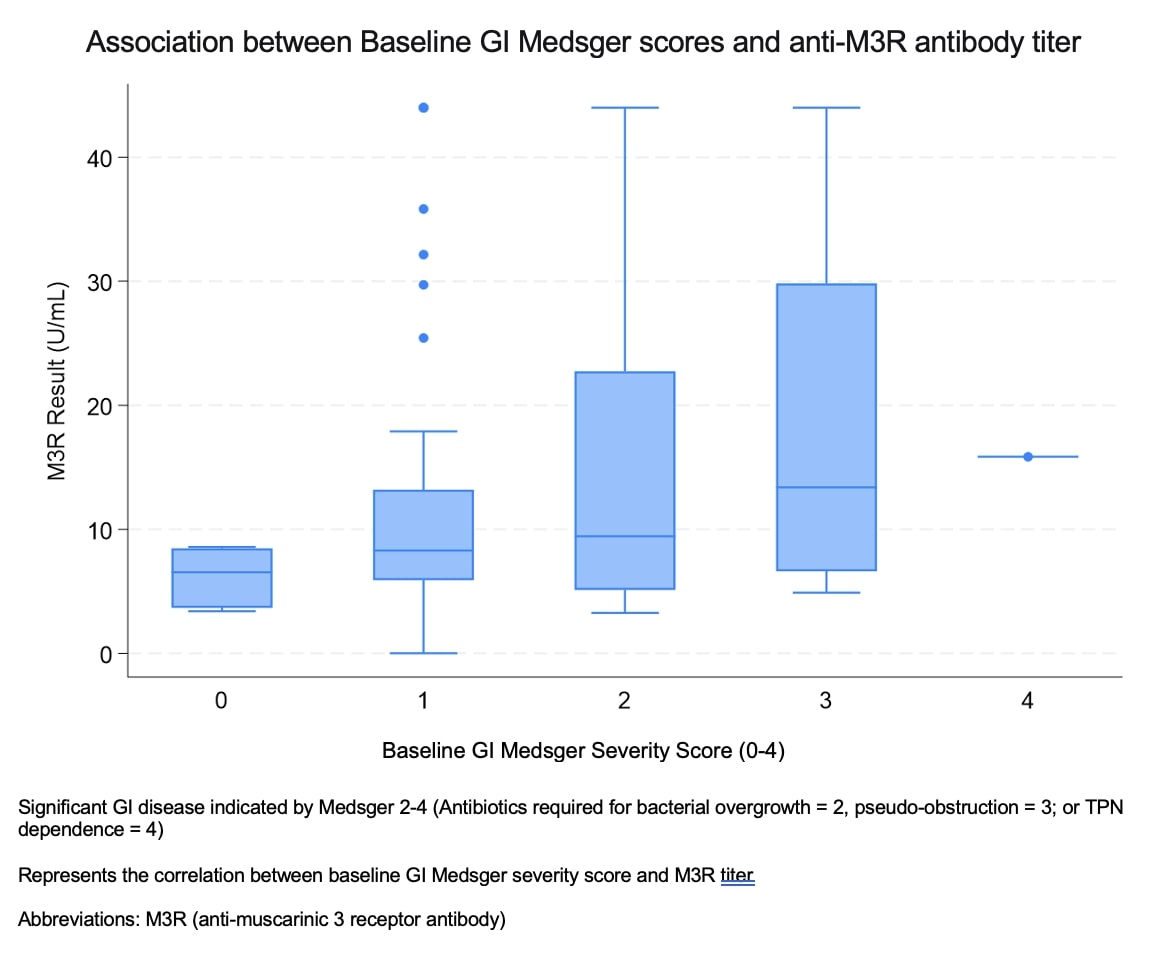
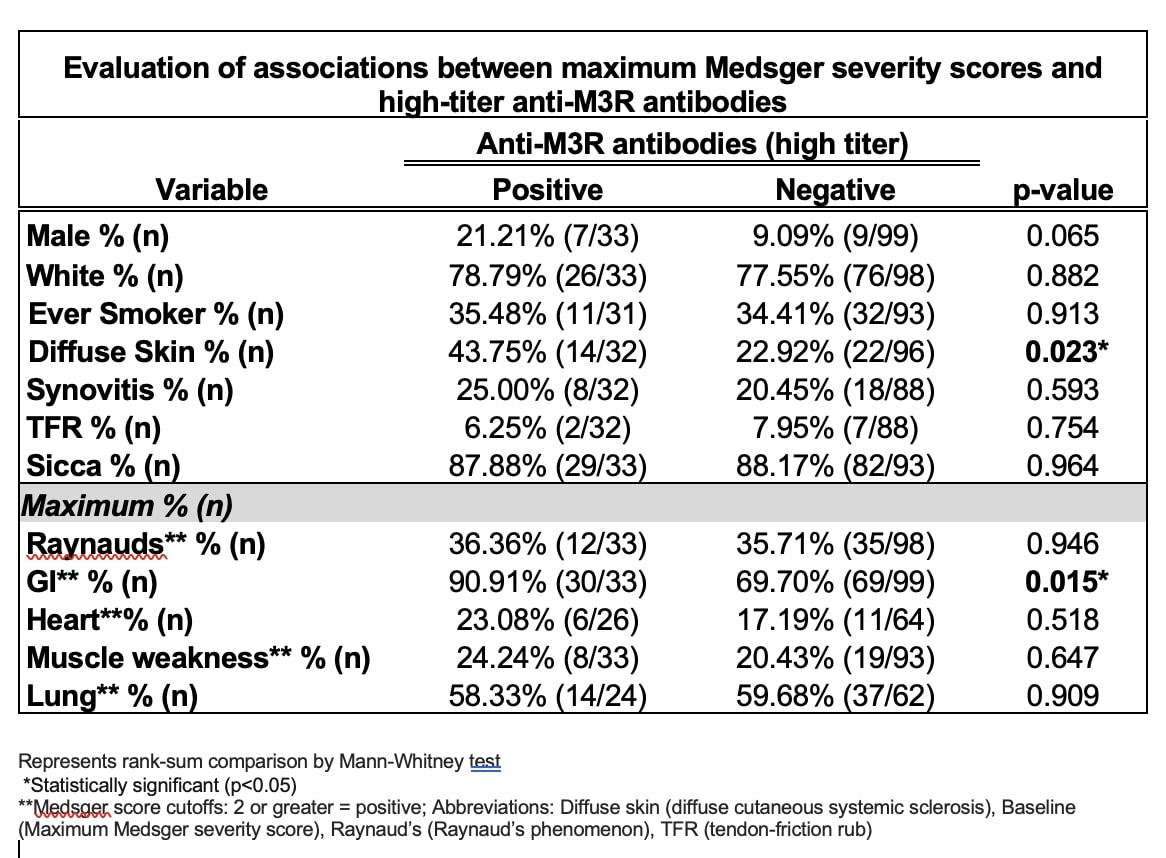
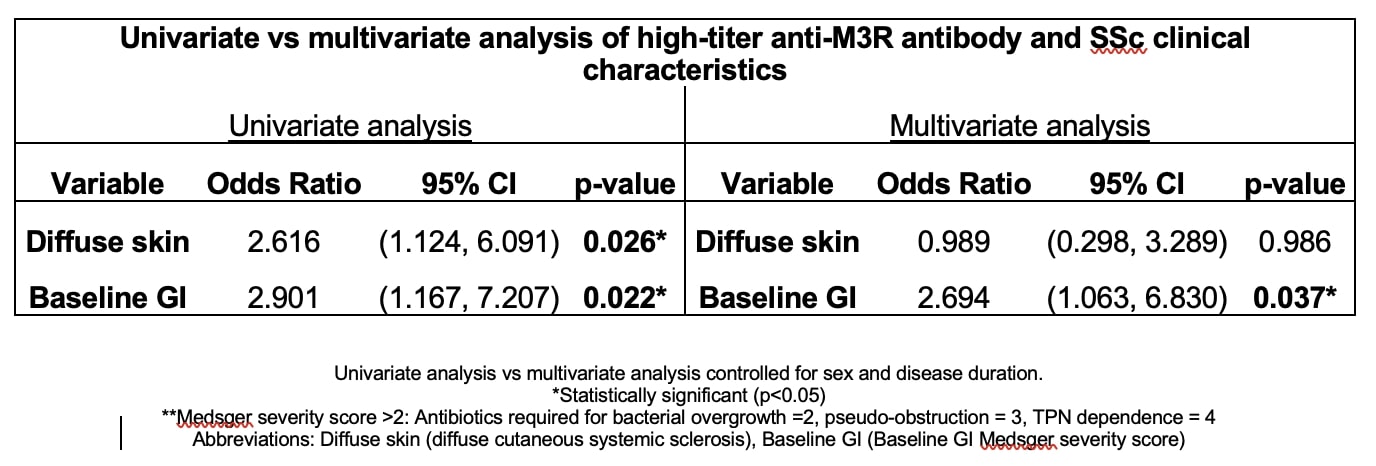
Results: Thirty-nine percent of patients (52/132) in this cohort were anti-M3R antibody positive according to the standard assay cutoff. Male sex (19% vs. 7.5%; p=0.044), synovitis (16% vs. 1.4%; p=0.003), and maximum Medsger GI scores ≥2 (88% vs. 66%; p=0.004) were significantly associated with anti-M3R antibodies in the univariate analysis. After adjusting for sex and disease duration in the multivariate model, anti-M3R antibody-positive patients were almost three times as likely to have significant baseline GI disease [OR 2.92, 95% CI (1.25, 6.83), p=0.014]. When evaluating patients with high-titer anti-M3R antibodies, 25% of patients were positive. High-titer anti-M3R antibodies were associated with more significant disease overall, which included diffuse (vs. limited) cutaneous disease (44% vs. 23%; p=0.023) and significant maximum GI symptoms (91% vs. 70% p=0.015). Subsequent multivariate analyses, adjusting for sex and disease duration, demonstrated that patients with high anti-M3R antibody titers were at least twice as likely to have significant baseline GI disease (OR 2.694, 95% CI (1.063, 6.830), p=0.037) compared to patients without high anti-M3R antibody titers.
Conclusion: Our data suggest that anti-M3R antibodies are associated with a specific clinical phenotype, including diffuse skin disease and significant baseline GI disease. Furthermore, significant antibody levels were associated with more significant GI and extraintestinal disease. Prospective and translational studies are needed to explore these important associations, as they may play a role in risk stratification and guide the management of patients with SSc.
To cite this abstract in AMA style:
Kalavar N, Hughes M, Morales W, Shah A, Volkmann E, Dongre R, Pimentel M, Hummers L, McMahan Z. Anti-muscarinic 3 Antibodies in SSc Associate with a More Significant GI and Extraintestinal Clinical Phenotype [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/anti-muscarinic-3-antibodies-in-ssc-associate-with-a-more-significant-gi-and-extraintestinal-clinical-phenotype/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/anti-muscarinic-3-antibodies-in-ssc-associate-with-a-more-significant-gi-and-extraintestinal-clinical-phenotype/