Session Information
Session Type: Poster Session A
Session Time: 8:30AM-10:30AM
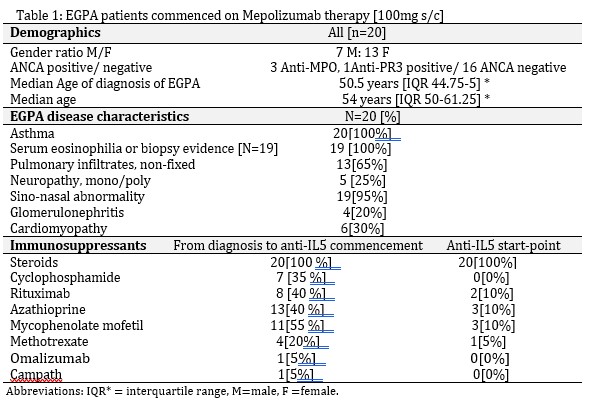
Background/Purpose: In the randomized, placebo-controlled MIRRA trial for relapsing and refractory eosinophilic granulomatosis with polyangiitis (EGPA), adjuvant therapy with 300mg anti-IL5 mAB Mepolizumab [MEPO] for 12 months (M), accrued longer times in remission, reduced steroid exposure and reduced relapse rates2. The aim of this study is to analyze the outcome of 100mg MEPO monthly s/c for a minimum of 24M in EGPA. Changes to adjuvant immunosuppression and indications for anti-IL5 class switch from MEPO 100mg s/c to Benralizumab (BRZ) or Reslizumab (Res) were assessed.
Methods: In this retrospective descriptive study, 20 EGPA patients received 100mg s/c MEPO every 4 weeks for a minimum of 24M (maximum duration 43M). Anti-IL5 therapy was switched between agents for poor response or intolerance. Time points of assessment included MEPO commencement, 6, 12, 18 and 24 months.
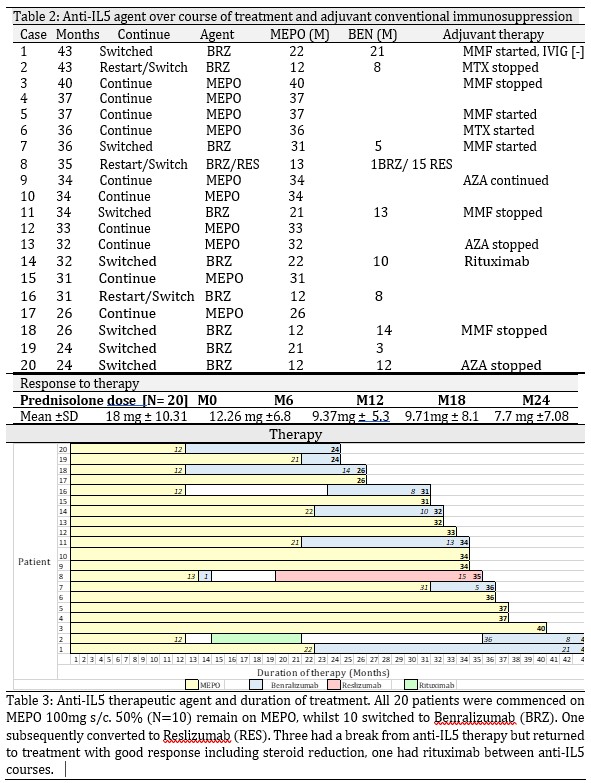
Results: Overall, there was a 50% reduction in steroid usage by 12 months. This continued to fall out to 24M. All 20 EGPA patients receiving anti -IL5 therapy, ranging from 24-43 months remain on therapy. 50% have remained on 100mg s/c MEPO and 50% have switched to an altenative anti-IL5 agent – 9 switched to benralizumab, 1 initially on benralizumab to reslizumab. All remain on anti-IL5 therapy after switch. Three patients had a break of therapy, but all resumed anti-IL5 treatment. 8 have anti-IL5 monotherapy as their maintenace regimen, an additional 6 weaned off conventional immunosuppressants. 6 continued or commenced conventional immunotherapy therapy which is well tolerated. ANCA serology normalized in all four positive patients by 12 months.
Conclusion: The relapsing nature of EGPA places a potential dependency of therapy on steroids, underscoring the importance of pathway specific biologics to minimize exposure, prevent tissue damage and ensure early response to therapy. There was a 50% reduction in steroid dosage in this study by 12 months and steroid requirements continue to decrease to 24 M. Longer-term anti-IL5 therapy is continuing in all 20 patients due to clinical benefits achieved. Anti-IL5 monotherapy is effective in some cases. Adjuvant immunotherapy is well tolerated and reduced in some cases. This study demonstrates that anti-IL5 therapy serves as a favorable model for steroid minimization in EGPA.
To cite this abstract in AMA style:
Egan A, Sivasothy P, Willcocks L, Jones R, Martinez Del Pero M, Jayne D. Anti-IL5 Therapy in Eosinophilic Granulomatosis with Polyangiitis (EGPA): A Longitudinal Follow-up Study ≥ 24months [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/anti-il5-therapy-in-eosinophilic-granulomatosis-with-polyangiitis-egpa-a-longitudinal-follow-up-study-%e2%89%a5-24months/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/anti-il5-therapy-in-eosinophilic-granulomatosis-with-polyangiitis-egpa-a-longitudinal-follow-up-study-%e2%89%a5-24months/