Session Information
Session Type: Poster Session
Session Time: 10:30AM-12:30PM
Background/Purpose: Refractory autoimmune diseases with progressive organ damage remain a major unmet medical need. C-CAR168 is an autologous anti-CD20/BCMA bispecific CAR-T therapy designed to simultaneously target autoantibody-producing plasma cells and their B-cell precursors, aiming to achieve a complete “immune reset”. This first-in-human Phase I trial (NCT06249438) evaluates C-CAR168 in patients with treatment-refractory autoimmune diseases. Here, we report the trial’s safety data and preliminary efficacy in the lupus nephritis (LN) and multiple sclerosis (MS) cohorts.
Methods: This Phase I, open-label study enrolled patients with refractory SLE (±LN), immune mediated necrotizing myositis (IMNM), neuromyelitis optica spectrum disorder (NMOSD), and MS, who had failed ≥2 standard immunosuppressive therapies. After lymphodepletion, patients received a single C-CAR168 infusion at either 0.75×10⁶ or 1.5×10⁶ CAR-T cells/kg.
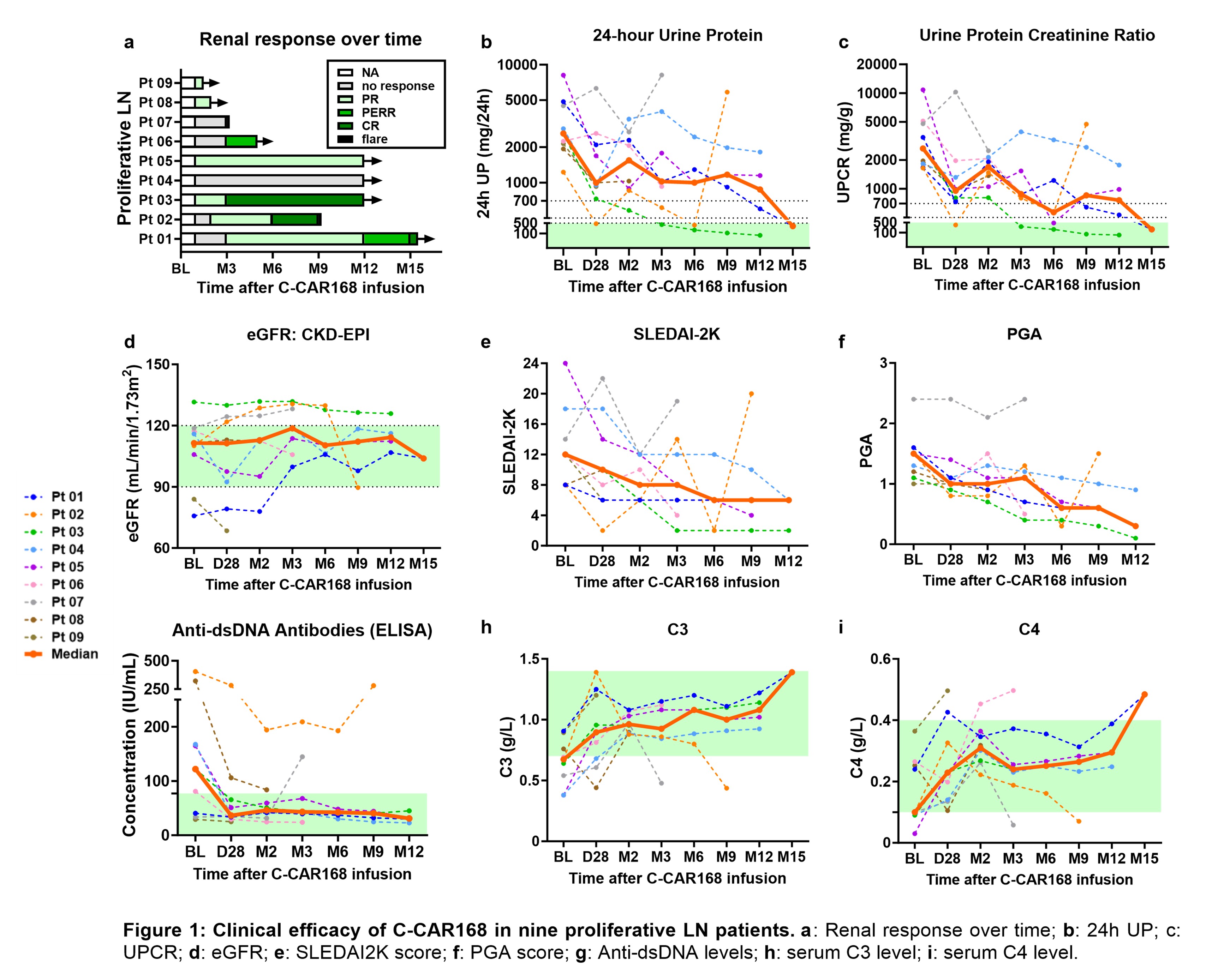
Results: As of September 10, 2025, 16 patients were treated: 11 SLE (9 proliferative LN), 2 NMOSD, 2 progressive MS and 1 IMNM, with a median follow-up of 177 days. C-CAR168 was well tolerated. Grade 1-2 CRS occurred in 11/16 patients (68.8%) and resolved with supportive care. No ICANS or DLTs were observed. Grade ≥3 AEs were primarily hematologic and transient. Prolonged cytopenia and Grade 3 treatment-emergent infections each occurred in 2 patients (12.5%) and were successfully managed with standard therapies. All patients discontinued immunosuppressants (IS) / biologic agents, 12 (75%) achieved steroid discontinuation and 4 remained on corticosteroids ≤10 mg/day.
Robust efficacy was observed in the LN and SPMS patients. In proliferative LN (median 4 prior IS/ biologic agents, excluding steroids/HCQ), 7/9 patients achieved a renal response (77.8%), including 3 CR, 1 PERR and 3PR. Improvement in proteinuria was rapid (median 1M [range 1–3]), and continued to improve with longer follow-up. Improvement in extrarenal SLE activity, as measured by SRI-4, was achieved in 6/7 (85.7%) proliferative LN patients with ≥3M follow-up. The first SPMS patient showed significant improvement in gait and orbital movement, EDSS score, reductions in MRI lesion burden, and normalization of serum neurofilament light chain.
C-CAR168 expansion was accompanied by rapid depletion of CD20⁺ B cells and plasma cells in blood, followed by naïve-dominant B-cell reconstitution in most patients by months 2-3, along with reductions in the type I IFN gene signature score. In one patient, bone marrow biopsy confirmed complete plasma cell depletion. Longitudinal examination of the B cell receptor repertoire (BCR) in 3 LN patients revealed a loss of clonally expanded BCRs with subsequent emergence of a naïve repertoire after treatment.
Conclusion: C-CAR168 therapy demonstrated durable treatment-free disease control in refractory autoimmune diseases. Its favorable safety profile, together with a renal response rate of 77.8% in refractory proliferative LN and rapid neurological improvement in SPMS, both achieved without background standard of care medications, coupled with mechanistic evidence of immune reset, provides a compelling rationale for further clinical evaluation of C-CAR168.
To cite this abstract in AMA style:
Ding H, Li W, Shen Y, Zhang C, Ye Y, Wang R, Yang S, Wu C, Dai D, Zheng C, Qian Y, Luo X, Trinh T, Zhu J, Huang J, Hao Y, Yao X, Ling Z, Yao Y, Shen N. Anti-CD20/BCMA Bispecific CAR-T Cell Therapy Promotes Immune Reset and Sustained Drug Free Remission in Refractory Autoimmune Diseases [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/anti-cd20-bcma-bispecific-car-t-cell-therapy-promotes-immune-reset-and-sustained-drug-free-remission-in-refractory-autoimmune-diseases/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/anti-cd20-bcma-bispecific-car-t-cell-therapy-promotes-immune-reset-and-sustained-drug-free-remission-in-refractory-autoimmune-diseases/

.jpg)
.jpg)