Session Information
Date: Monday, November 18, 2024
Title: SLE – Treatment Poster III
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Anti-CD19 chimeric antigen receptor (CAR) T cells have emerged as a promising therapeutic option for treatment-refractory patients with B cell-mediated diseases such as systemic lupus erythematosus (SLE) (Mackensen A et al. Nat Med 2022, Müller F et al. NEJM 2024). Anti-CD19 CAR T cells trigger a rapid B cell depletion in the peripheral blood and simultaneously induce clinical remission. However, the effect of anti-CD19 CAR-T cell therapy in the bone marrow and lymph nodes of SLE patients has not yet been investigated.
Methods: A 46-year-old male patient with severe, treatment-refractory SLE underwent autologous anti-CD19 CAR T cell therapy with KYV-101 following lymphodepletion with fludarabine (15mg/m2 due to impaired kidney function) and cyclophosphamide (300mg/m2). Whole blood samples were obtained before and after anti-CD19 CAR T cell therapy. Additionally, bone marrow and inguinal lymph node biopsies were performed 16 weeks post-treatment. B and T cell immune phenotyping were conducted using flow cytometry.
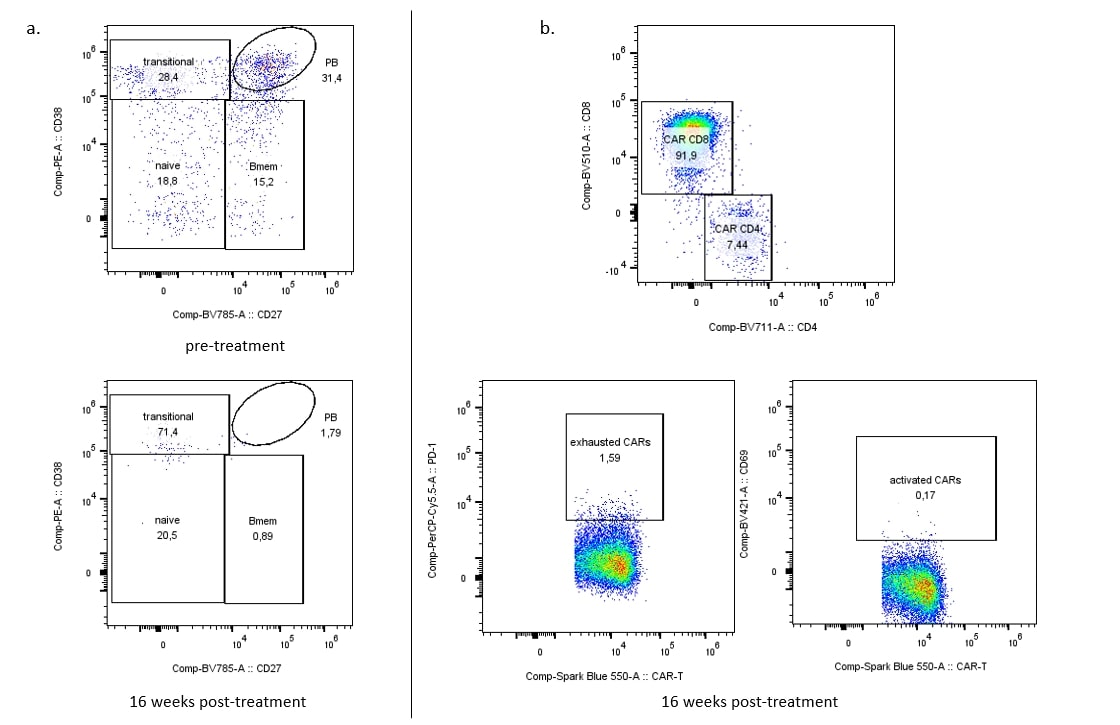
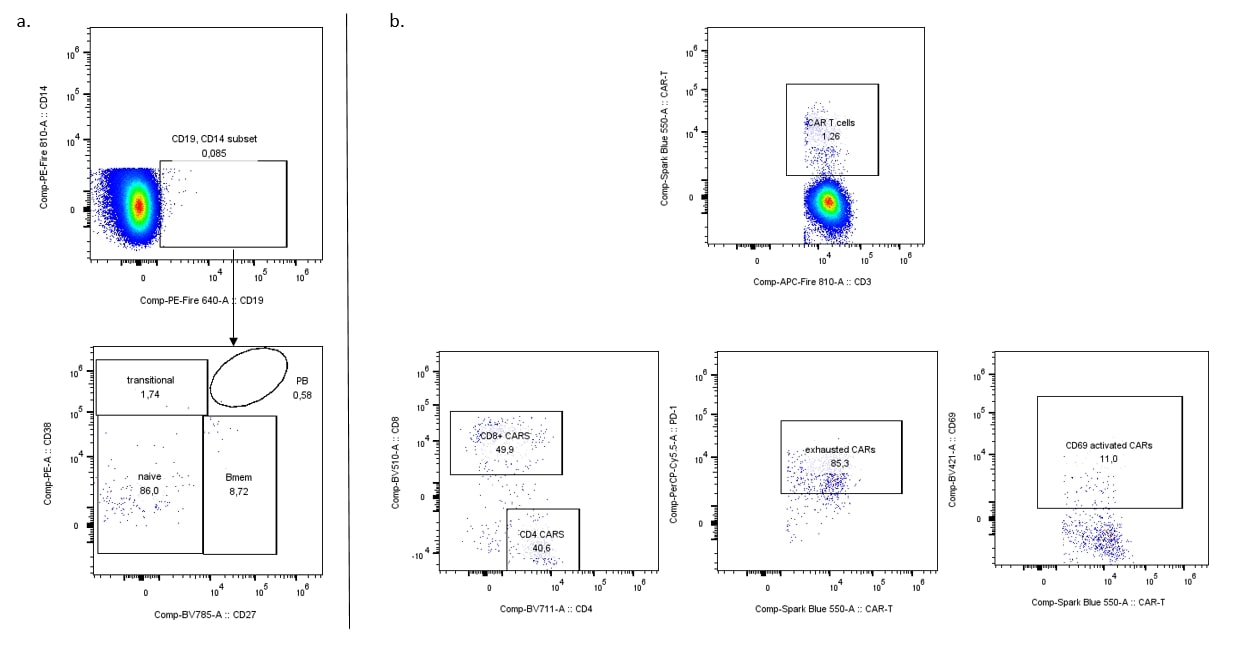
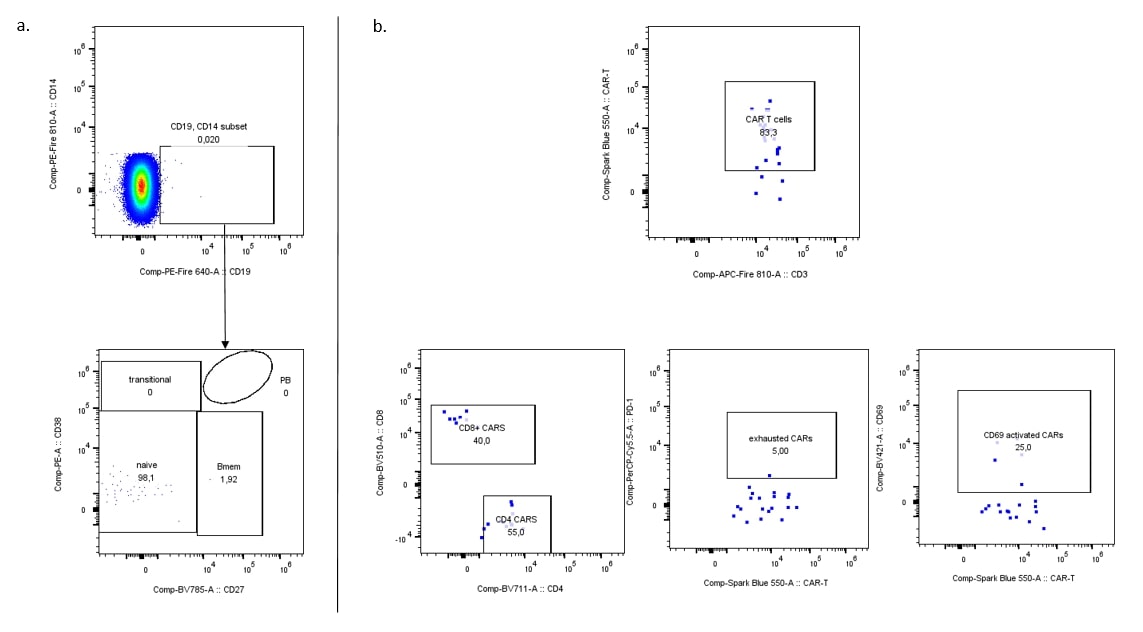
Results: Significant clinical improvement was observed 16 weeks after anti-CD19 CAR T cell infusion (SLEDAI 2K score reduction of 10 points from baseline), despite cessation of other immunosuppressive therapies. Peripheral blood B cells dramatically decreased and remained absent through week 16 post-treatment (Figure 1a) while anti-CD19 CAR T cells remained detectable (Figure 1b). Similarly, B cells and particularly CD19+ plasmablasts and CD19+ plasma cells in the bone marrow were significantly diminished at week 16, with the few detected B cells exhibiting a naïve phenotype (CD27–CD38– B cells) (Figure 2a). Anti-CD19 CAR T cells persisted in the bone marrow, where we observed an even distribution between CD4+ and CD8+ CAR T cells. Most of them exhibited high PD-1 expression, indicating exhaustion (McLane L et al. Annu Rev Immunol 2019) (Figure 2b). No B cells were detected in the lymph node at week 16 post-treatment (Figure 3a) and anti-CD19 CAR T cells were also rare (Figure 3b).
Conclusion: In SLE, anti-CD19 CAR T cell therapy enables broad, tissue-based depletion of B cells in the peripheral blood, bone marrow and lymph nodes 16 weeks post-treatment. These findings support the notion that anti-CD19 CAR T cell therapy induces remission in patients with SLE through complete and sustained depletion of autoreactive B cells within primary and secondary lymphatic organs.
To cite this abstract in AMA style:
Minopoulou I, Penack O, Albach F, Wilhelm A, Kleyer A, Borie D, Casteleyn V, Biesen R, Enghard P, Dörner T, Drzeniek N, Zernicke J, Alexander T, Movassaghi K, Hütter-Krönke M, Schrezenmeier E, Schreiber A, schneider u, Bullinger L, Krönke G, Simon D. Anti-CD19 Chimeric Antigen Receptor T Cell Therapy Induces Multicompartmental B Cell Depletion in Peripheral Blood, Bone Marrow and Lymph Nodes of Systemic Lupus Erythematosus [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/anti-cd19-chimeric-antigen-receptor-t-cell-therapy-induces-multicompartmental-b-cell-depletion-in-peripheral-blood-bone-marrow-and-lymph-nodes-of-systemic-lupus-erythematosus/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/anti-cd19-chimeric-antigen-receptor-t-cell-therapy-induces-multicompartmental-b-cell-depletion-in-peripheral-blood-bone-marrow-and-lymph-nodes-of-systemic-lupus-erythematosus/