Session Information
Date: Saturday, November 16, 2024
Title: SpA Including PsA – Diagnosis, Manifestations, & Outcomes Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Human leukocyte antigen (HLA)-B27 is linked to spondyloarthropathies (SpA), uveitis, and inflammatory bowel disease (IBD). By analyzing ordering diagnoses and final diagnoses during patient follow-up, we aim to look at cost utilization and yield of diagnosis for different departments to improve appropriateness of HLA-B27 testing for diagnosis in a rural academic health system.
Methods: This is a retrospective study from January 2018 to July 2022, analyzing the electronic health record for HLA-B27 tests, together with associated ordering diagnoses and department of ordering provider. A random manual chart review was done for 1400 charts among initial sample of 3400, confirming ordering diagnosis and final diagnosis at follow up. The ordering diagnoses and final diagnosis were categorized as appropriate or non-appropriate, with appropriate defined by diagnosis related to HLA-B27 positivity, such as ankylosing spondylitis, psoriatic arthritis, uveitis, IBD, juvenile arthritis, and reactive arthritis. Ordering providers were grouped into subspecialities, specialized in treating these HLA-B27 appropriate diagnosis (e.g. Rheumatology, Dermatology, Ophthalmology, and Gastroenterology) and all other ordering providers within our system.
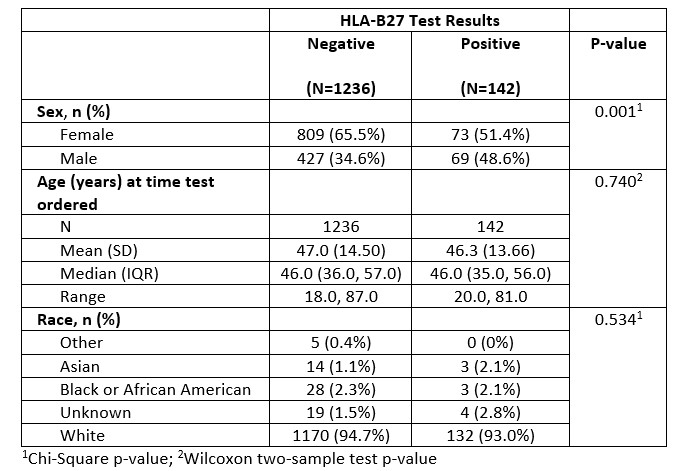
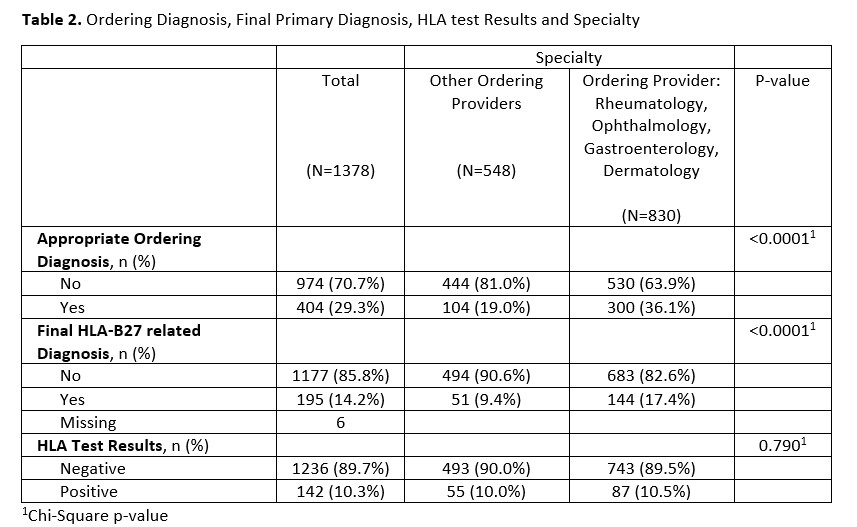
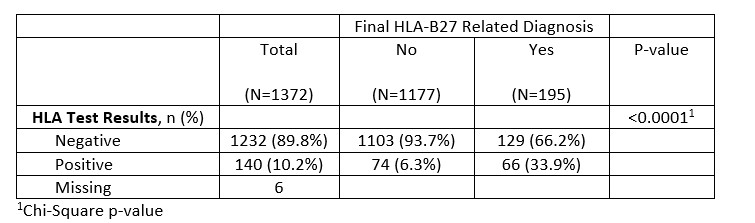
Results: Of the 1400 charts, after excluding patients with indeterminate HLA test result, and subsequent encounter for duplicate charts, a final sample size of 1378 distinct charts were analyzed. Of the reviewed HLA-B27 orders, 89.7% of tests were negative, while 10.3% of tests were positive. A higher percentage of patients whose test was ordered by primary 4 subspecialities had ordering diagnosis appropriate for HLA-B27 compared to patients whose test was ordered by other providers (36.1% vs 19.0%, p-value < 0.0001). In addition, a higher percentage of patients whose test was ordered by a provider in the subspecialty group had a final diagnosis appropriate to HLA-B27 positivity (17.4% vs 9.4%, p-value < 0.0001).
Among the 830 patients tested by subspecialties, 10.5% had positive test and 17.4% were diagnosed with HLA-B27 associated diseases. In contrast, of the 548 patients tested by others, 10% had a positive result, and only 9.4% were diagnosed with HLA-B27 associated disease (p< 0.0001).
The cost of testing at our center is $447. The total cost for 1400 patients was $625,800, of which $435,378 was spent on ordering diagnosis not related to HLA-B27 related disease. For subspecialities, the overall cost was $371,010, of which $64,368 were used for patients diagnosed with HLA B27 related disease. For other providers, the overall cost was $244,956 and $22,797 used for patients diagnosed with HLA-B27 disease.
Conclusion: In our rural health system, there is an excessive utilization of HLA-B27 testing across all providers. Rheumatology, Dermatology, Ophthalmology, and Gastroenterology have more appropriate ordering diagnosis and had significantly more patients ultimately diagnosed with HLA-B27 related diseases. We intend to implement guidance for providers on the utilization of HLA-B27 to reduce extraneous orders.
To cite this abstract in AMA style:
Shrestha S, Bobak A, Udoeyo I, Puri P, Law J, Bulbin D. Analyzing the Utilization of HLA-B27 Testing in a Large Rural Health System: Implications for Diagnostic Appropriateness [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/analyzing-the-utilization-of-hla-b27-testing-in-a-large-rural-health-system-implications-for-diagnostic-appropriateness/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/analyzing-the-utilization-of-hla-b27-testing-in-a-large-rural-health-system-implications-for-diagnostic-appropriateness/