Session Information
Date: Sunday, November 7, 2021
Session Type: Poster Session B
Session Time: 8:30AM-10:30AM
Background/Purpose: Longer disease duration and greater number of prior DMARDs have been associated with lower treatment efficacy in patients with RA.1 Abatacept is a biologic (b)DMARD for treatment of moderate-to-severe RA and is available in SC formulation, which may offer convenience benefits with efficacy similar to IV administration.2 ASCORE (NCT02090556) was a 2-year, observational, prospective, multicenter study of SC abatacept for treatment of RA in routine clinical practice.3 This post hoc analysis was conducted to determine if retention and efficacy of abatacept were impacted by disease duration and/or treatment line.
Methods: Eligible patients, aged ≥18 years, with active moderate-to-severe RA (ACR/EULAR 2010 criteria4) who were IV abatacept-naive and initiated SC abatacept 125 mg once weekly, were enrolled into 2 cohorts: bDMARD-naive patients and those with ≥1 prior bDMARD treatment failure. This post hoc analysis evaluated abatacept retention using Kaplan-Meier estimates, as well as disease activity scores (DAS28 [ESR]), CDAI and SDAI in patients with disease duration of ≤2, 3–5, 6–10 or >10 years, and in patients taking abatacept as first-line or ≥ second-line treatment.
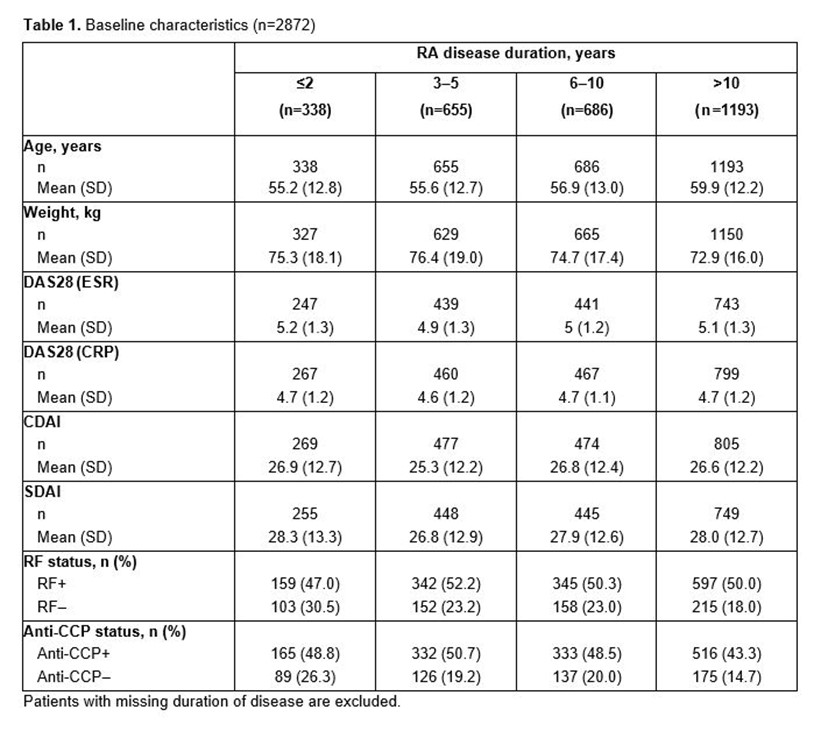
Results: Table 1 shows baseline characteristics. Mean age increased with disease duration; other characteristics were comparable across groups. Retention proportions (95% CIs) at Month 24 were 0.50 (0.4, 0.5), 0.47 (0.4, 0.5), 0.51 (0.5, 0.5) and 0.46 (0.4, 0.5) in the ≤2, 3–5, 6–10 and >10 years’ duration groups, respectively. Proportion of patients (95% CI) with ≤2 years’ duration retaining treatment at Month 24 were 0.51 (0.4, 0.6) among those using abatacept as first-line treatment and 0.44 (0.3, 0.6) among those using abatacept as a ≥ second-line treatment (Figure 1). Proportions (95% CI) at Month 24 were 0.51 (0.5, 0.6), 0.57 (0.5, 0.6) and 0.52 (0.5, 0.6) in first-line patients and 0.43 (0.4, 0.5), 0.48 (0.4, 0.5) and 0.44 (0.4, 0.5) in ≥ second-line patients in the 3–5, 6–10 and >10 years’ duration groups, respectively. Mean (SE) changes from baseline in DAS28 (ESR) at Month 24 were –2.12 (0.205), –1.86 (0.151), –2.07 (0.140) and –2.05 (0.115) in the ≤2, 3–5, 6–10 and >10 years’ duration groups, respectively; respective mean (SE) changes in CDAI were –18.74 (1.604), –15.60 (1.099), –18.50 (1.038) and –17.68 (0.850); and respective mean (SE) changes in SDAI were –19.10 (1.873), –15.72 (1.345), –19.54 (1.103) and –17.07 (0.939).
Conclusion: In this post hoc analysis of the real-world ASCORE trial, patients with RA receiving abatacept in clinical practice as first-line therapy had better retention versus those receiving it as a ≥ second-line treatment, regardless of disease duration at baseline. Retention rates were similar across disease duration subgroups. Improvements in disease activity were seen in all duration subgroups, without consistently greater or lesser improvement seen with longer disease duration.
References:
1. Aletaha D, et al. Ann Rheum Dis 2019;78:1609–1615.
2. Genovese MC, et al. Arthritis Rheumatol 2011;63:2854–2864.
3. Alten R, et al. Ann Rheum Dis 2019;78(suppl 2):A1639.
4. Aletaha D, et al. Arthritis Rheum 2010;62:2569–2581.
Medical writing: Robert Coover (Caudex)
Original abstract © 2020 EULAR/BMJ
To cite this abstract in AMA style:
Alten R, Mariette X, Flipo R, Caporali R, Buch M, Patel Y, Sanmarti R, Marsal S, Nurmohamed M, Griffiths H, Peichl P, Bannert B, Forster A, Chartier M, Elbez Y, Rauch C, Khaychuk V, Lozenski K. Analysis of Abatacept Treatment Retention and Efficacy According to Disease Duration and Treatment Line in a Real-World Setting [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/analysis-of-abatacept-treatment-retention-and-efficacy-according-to-disease-duration-and-treatment-line-in-a-real-world-setting/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/analysis-of-abatacept-treatment-retention-and-efficacy-according-to-disease-duration-and-treatment-line-in-a-real-world-setting/