Session Information
Date: Monday, November 8, 2021
Session Type: Poster Session C
Session Time: 8:30AM-10:30AM
Background/Purpose: Previous studies have demonstrated that metabolic factors may predispose to giant cell arteritis (GCA). The purpose of this study was to investigate the relation between biomarkers of inflammation in biobank samples and subsequent development of GCA.
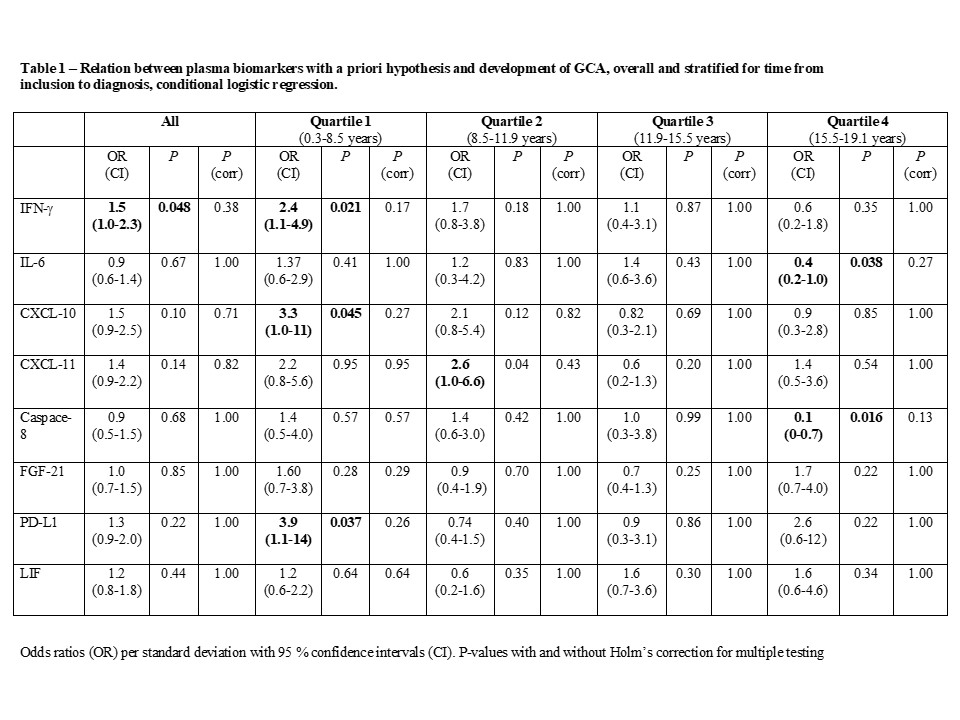
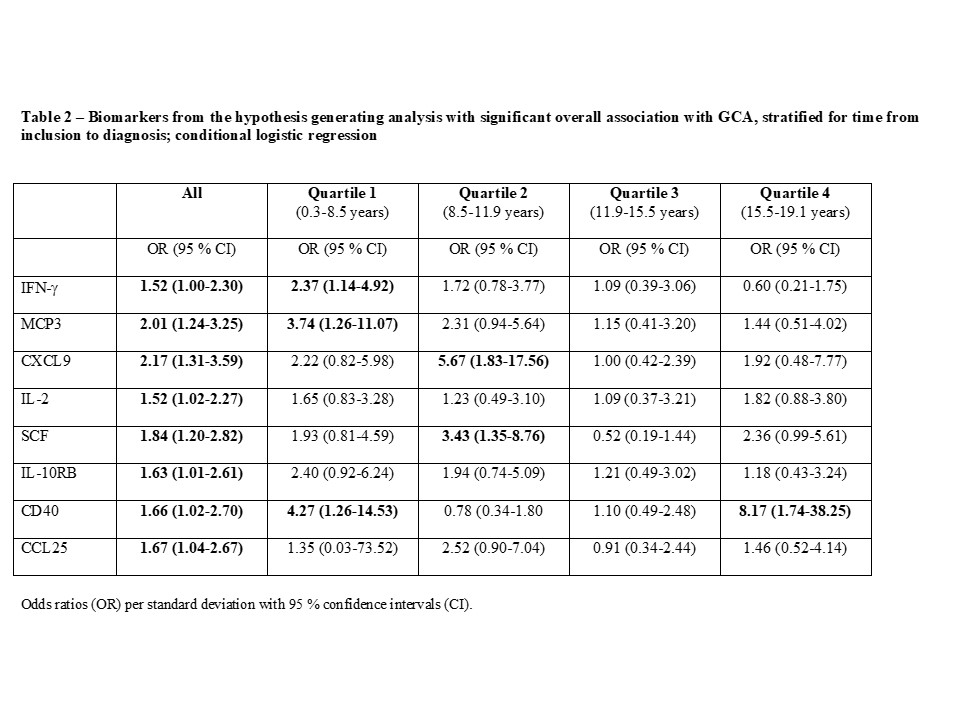
Methods: Participants in a population-based cohort established in 1991-1996 who were subsequently diagnosed with GCA were identified through register linkage and validated in a structured process. GCA-free controls, matched for sex, year of birth, and year of inclusion, were selected from the study cohort. Plasma samples from cases and controls that had been obtained at inclusion were analyzed using the OLINK proteomics inflammation panel, which measures 92 inflammatory proteins, and provides arbitrary values based on log2 normalized quantification. Variables with a skewed distribution were log transformed, and Z-scores for all proteins were included in conditional logistic regression models with GCA case/control status as the dependent variable. Analyses were pre-designated as hypothesis-driven or hypothesis-generating. A priori hypotheses were formulated for 8 biomarkers. For these, Holm’s correction of p-values was applied to account for multiple testing. In the hypothesis-generating part of the study, principal component analysis was used to identify groups of proteins that explain the variance in the proteome. Within components selected based on Eigenvalues, proteins with a factor loading of >0.50 were investigated as potential predictors of GCA. All analyses were stratified by quartile of time from inclusion to GCA diagnosis among the cases.
Results: A total of 94 cases with a confirmed incident diagnosis of GCA (82 % female; 64 % biopsy positive, mean age at diagnosis 73.6 years) were identified and had preserved plasma samples. The median time from inclusion to diagnosis was 11.9 years (range 0.3-19.1). Among biomarkers with a priori hypotheses, IFN-g was positively associated with GCA (odds ratio (OR) 1.52; 95% confidence interval (CI) 1.00-2.30). However, none of the associations in this subset reached statistical significance after correction for multiple testing (Table 1). Of 37 biomarkers with factor loading >0.5 within 6 principal components with Eigenvalues >2.5, 8 were significantly associated with development of GCA (Table 2). Among these, higher levels of IFN-g (OR 2.37; 95% CI 1.14-4.92) and MCP3 (OR 4.27; 95% CI 1.26-14.53) were particularly associated with increased risk of GCA in the subset sampled < 8.5 years before diagnosis. Several other proteins known to be important for T cell function were also associated with GCA in these analyses, e.g. CXCL9, IL-2, CD40 and CCL25 (Table 2).
Conclusion: In this nested-case control study, elevated IFN-g levels were found years prior to diagnosis in cases who were subsequently diagnosed with GCA, although we cannot exclude that this finding is due to multiple testing. Principal component analysis revealed that several T cell related proteins that explain a major part of the variance in the proteome were associated with subsequent GCA, suggesting that T cell activation may precede the clinical onset of GCA.
To cite this abstract in AMA style:
Wadström K, Nilsson J, Mohammad A, Warrington K, Matteson E, Jakobsson M, Jacobsson L, Turesson C. Analyses of Plasma Inflammatory Proteins Reveal Biomarkers Predictive of Subsequent Development of Giant Cell Arteritis; A Nested Case-Control Study [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/analyses-of-plasma-inflammatory-proteins-reveal-biomarkers-predictive-of-subsequent-development-of-giant-cell-arteritis-a-nested-case-control-study/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/analyses-of-plasma-inflammatory-proteins-reveal-biomarkers-predictive-of-subsequent-development-of-giant-cell-arteritis-a-nested-case-control-study/