Session Information
Session Type: Abstract Session
Session Time: 1:00PM-2:30PM
Background/Purpose: Hand osteoarthritis is a debilitating and highly prevalent disease with limited treatment options. The aim of this study was to investigate the efficacy and safety of a newly developed combined supplement in people with hand osteoarthritis (OA).
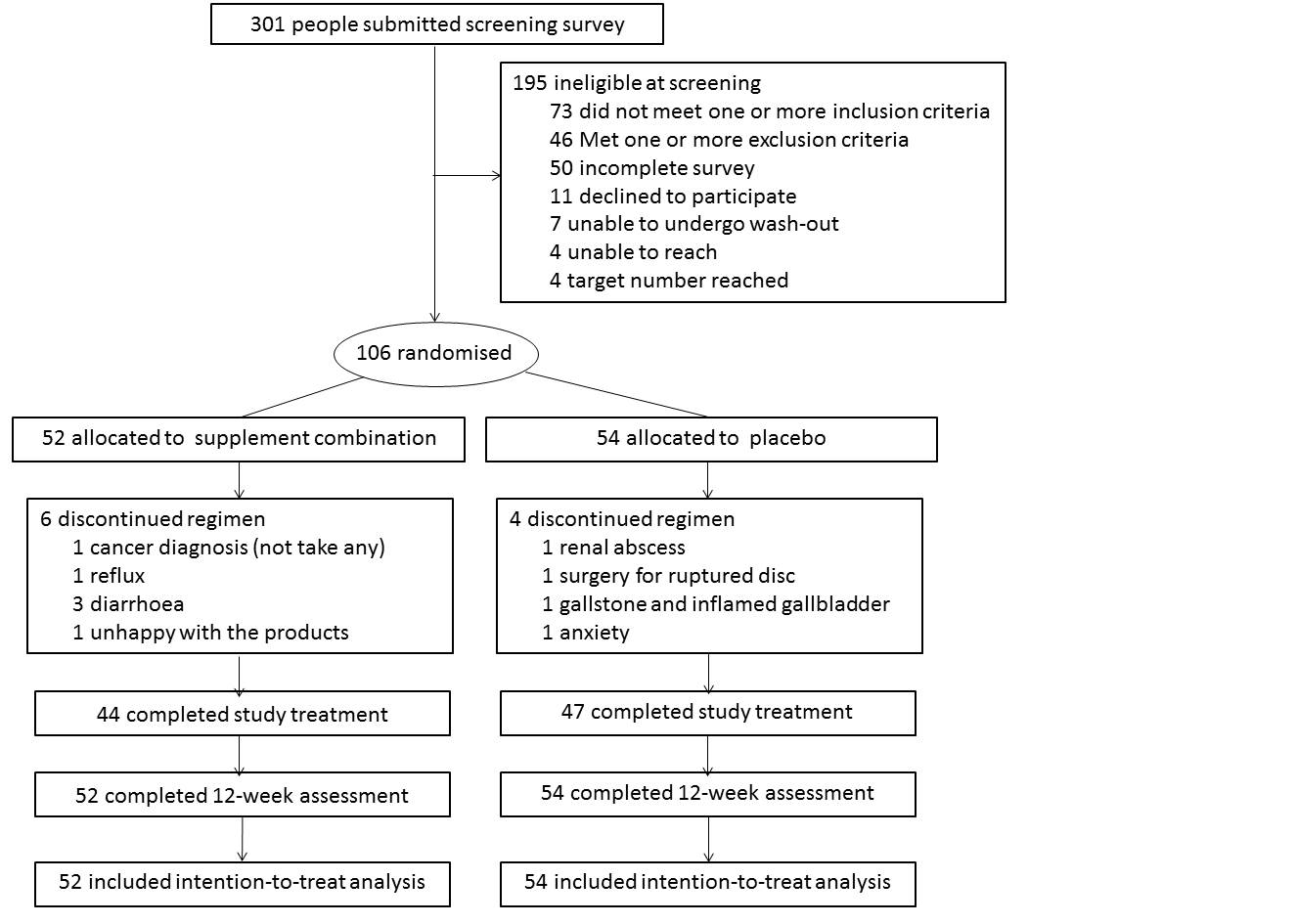
Methods: This was an internet-based, double-blind, randomised, placebo-controlled trial without face-to-face interaction between investigators and participants. The main eligibility criteria were aged over 40 years with symptomatic hand OA and Kellgren Lawrence grade (KLG) 2 and above. The recruitment was conducted across Australia. Eligible participants were randomly assigned to receive either a combined supplement or placebo (1:1) for 12 weeks. The active ingredients in the combined supplement were Boswellia serrata extract 250 mg/day, pine bark extract 100 mg/day, methylsulfonylmethane 1,500 mg/day and curcumin 168 mg/day. The primary outcome was change in hand pain assessed using a visual analogue scale (VAS, 0-100) from baseline to week 12. Secondary outcomes included change in functional Index of Hand Osteoarthritis, patient global assessment and health-related quality of life. Adverse events were monitored weekly.
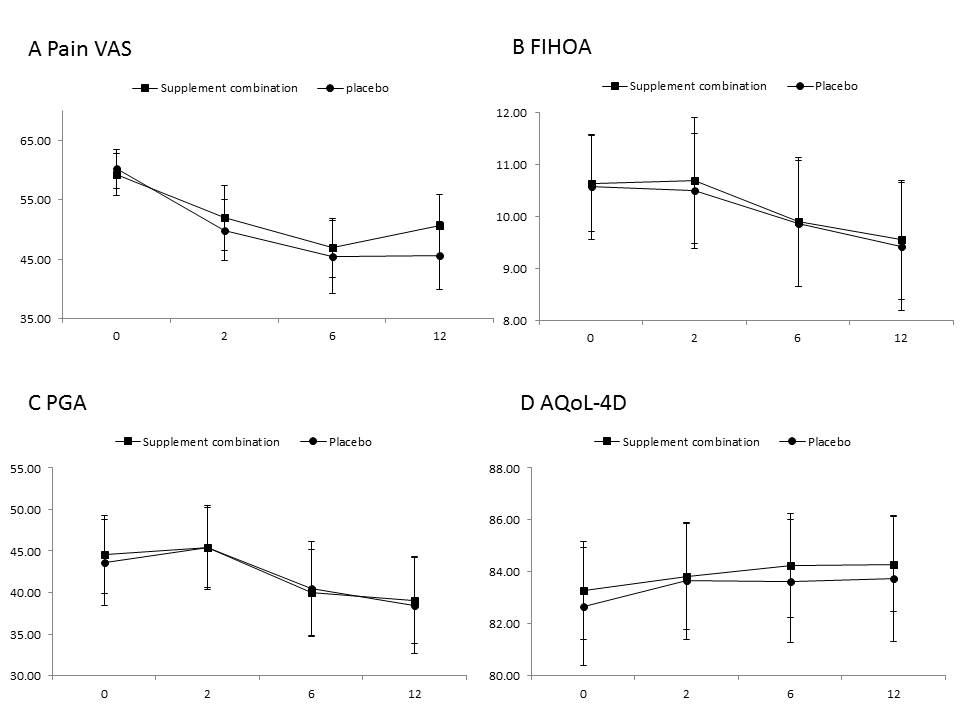
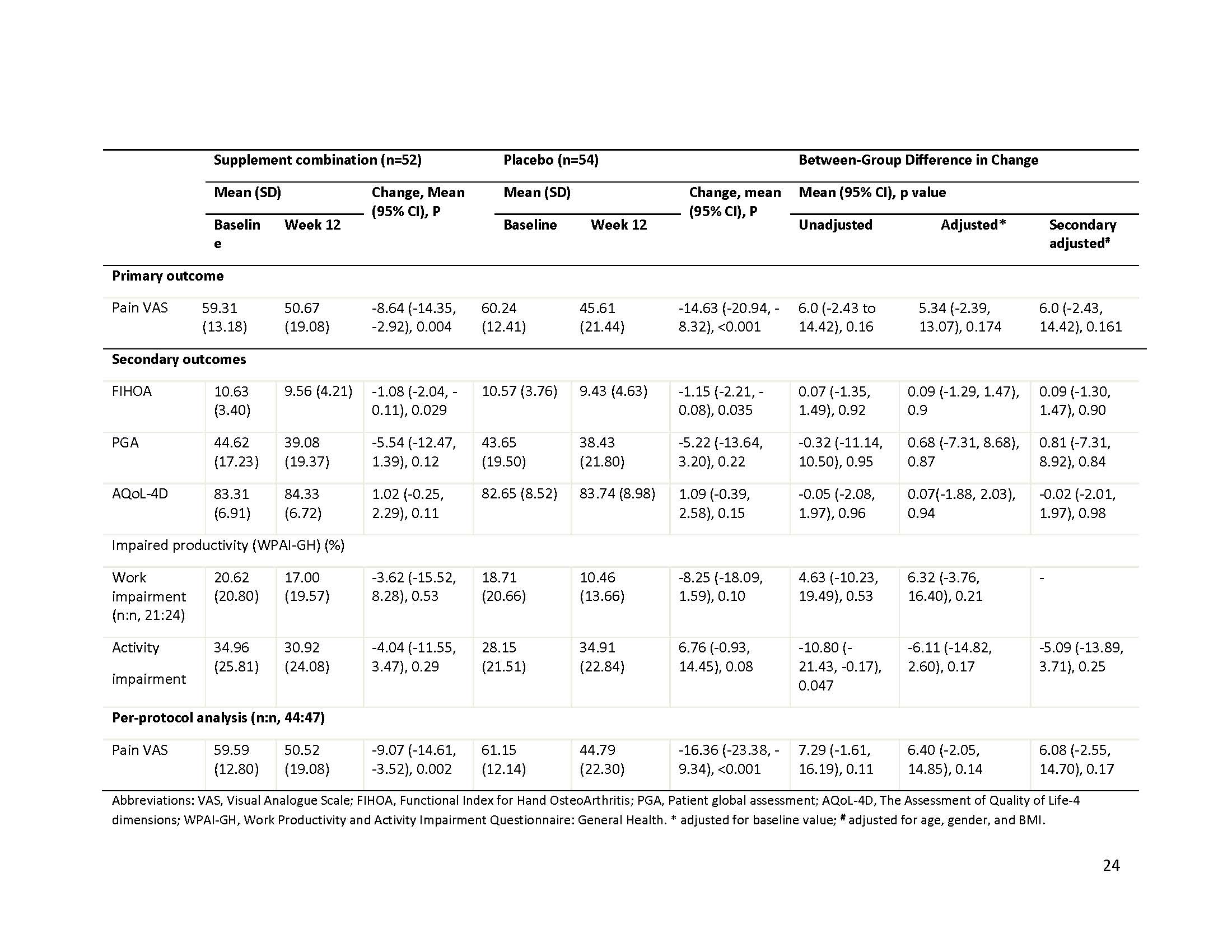
Results: We included a total of 106 participants with a mean age of 65.6 years and 81% female. The majority of the participants were moderate to severe on the radiographic assessment, with 40% KLG 3 and 45% KLG 4, and 37% participants had erosive OA. Pain VAS decreased over 12 weeks in both active and placebo groups with mean change (95%CI) -8.64 (-14.35 to -2.92, p=0.004) and -14.63 (-20.94 to -8.32, p < 0.001), respectively. The adjusted between-group difference over 12 weeks was 5.34 (95%CI, -2.39 to 13.07, p=0.17). Five participants (10%) in the supplement combination group discontinued study treatment due to AE vs four participants (7%) in the placebo group.
Conclusion: Treatment with the supplement combination was not superior to treatment with a placebo for improving hand pain over 12 weeks. Results do not support the initiation of supplement combination therapy for people with moderate- to late-stage- hand OA.
To cite this abstract in AMA style:
Liu X, Robbins S, Eyles J, Deveza L, McLachlan A, Hunter D. An Online Trial to Assess the Efficacy and Safety of a Supplement Combination in People with Hand Osteoarthritis [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/an-online-trial-to-assess-the-efficacy-and-safety-of-a-supplement-combination-in-people-with-hand-osteoarthritis/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/an-online-trial-to-assess-the-efficacy-and-safety-of-a-supplement-combination-in-people-with-hand-osteoarthritis/