Session Information
Date: Monday, October 27, 2025
Title: (1147–1190) Miscellaneous Rheumatic & Inflammatory Diseases Poster II
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: The development of advanced therapies such as JAK inhibitors (JAKi) has expanded treatment options available for patients with rheumatic and musculoskeletal diseases (RMDs). Case reports and scientific studies are increasingly demonstrating the potential scientific rationales regarding JAKi use for broader indications. Defining current and anticipated prescribing habits may help predict the future treatment paradigm and understand key evidence gaps. This study aimed to capture global discussions on the evolution of JAKi use within (1) current indications and (2) new indications where JAKi may have potential place in therapy as identified by unmet medical need, external global expert opinion and literature evidence using a modified Delphi approach.
Methods: A 4 round modified Delphi study was conducted with 178 Panellists from 23 countries worldwide. Global experts formed the academic and steering committees. Systematic and scoping reviews on published literature of JAKi use in RMDs were conducted to inform initial statements alongside committee expertise. Panellists ranked statements on a Likert scale of 1-9, provided comment on statement stringency and suggestions. Stable statement consensus or stable disagreement was considered as a median score of ≥7 or ≤3 from two consecutive rounds, respectively. Statements were presented in ≥2 rounds and wording amended based on panellist suggestions, confirmed by committees.
Results: Panellists (n=178) participated from: Americas (n=30), Europe (n=40), Eastern Mediterranean (n=35), South-East Asia (n=19), Africa (n=27), and Western Pacific (n=27) (Figure 1). Statements were grouped into the following categories: Current Uses of JAKi, Potential uses of JAKi beyond currently approved indications, Potential uses of a specific class of JAKi: TYK2 inhibitors or Acquisition and access to JAKis (Figure 2/Table 1). Of the 79 statements presented, 20 reached stable consensus, and one reached stable disagreement, with >250 comments. Consistent themes included the important role of JAKi in current indications, that the availability of generic JAKi may lead to their wider use, lack of RCT data on potential new indications and accessibility. Opinion stratification across key differentiators including geography, national income and treatment practice highlighted great heterogeneity in responses, particularly in relation to cost, JAKi adoption in earlier stages of indicated conditions, and potential expansion of their use more broadly.
Conclusion: Based on available literature evidence and global expert consensus, this study highlights global consensus characterization of current uses and the evolution of JAKi use in RMDs. Clinicians were in consensus that JAKi have an important role in the management of current indications. Additionally, opinion showed availability of generic JAKi may lead to their wider use and may broaden the indications for which they are utilised.This study was sponsored by Pfizer. Pfizer employees and academic authors designed the study, interpreted data, and wrote the abstract. Analytical and writing support was provided by Momentum data Ltd, funded by Pfizer. Some study elements previously accepted by BSR & EULAR 2025.
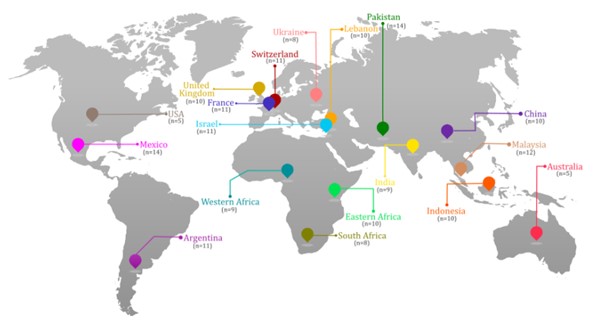 Figure 1: Global, geographical distribution of panellists
Figure 1: Global, geographical distribution of panellists
.jpg) Table 1: Statements which achieved stable consensus or stable disagreement regarding JAKi use in RMDs.
Table 1: Statements which achieved stable consensus or stable disagreement regarding JAKi use in RMDs.
.jpg) Figure 2: Detailed outcomes for statements achieving stable consensus in the overall global analysis.
Figure 2: Detailed outcomes for statements achieving stable consensus in the overall global analysis.
To cite this abstract in AMA style:
Barkaway A, Mease P, Rutter-Locher Z, Moots R, Ndosi M, McLean M. An International modified Delphi Study on the evolving role of Janus kinase inhibitors (JAKi) in rheumatic and musculoskeletal diseases (RMDs) [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/an-international-modified-delphi-study-on-the-evolving-role-of-janus-kinase-inhibitors-jaki-in-rheumatic-and-musculoskeletal-diseases-rmds/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/an-international-modified-delphi-study-on-the-evolving-role-of-janus-kinase-inhibitors-jaki-in-rheumatic-and-musculoskeletal-diseases-rmds/
