Session Information
Session Type: Abstract Session
Session Time: 9:00AM-10:30AM
Background/Purpose: There is evidence that extending the interval between COVID-19 vaccination doses is associated with increased immunogenicity and neutralizing activity in healthy individuals (1, 2). Previously, we reported higher humoral immune responses in patients with inflammatory rheumatic diseases (IRD) receiving mRNA-1273 vs BNT162b2 for their 2-dose primary immunization series (3). The same patients subsequently received a 3rd COVID-19 vaccine dose at varying intervals from the 2nd dose. The objectives of this study were to assess whether the interval or vaccine type (BNT162b2 vs mRNA-1273) affect the anti-SARS-CoV-2 Spike IgG levels up to 180 d post 3rd vaccine dose and the hazard of a positive SARS-CoV-2 test within 365 d post 3rd vaccine dose.
Methods: Patients from the Swiss cohort for patients with IRD (SCQM) who participated in the earlier phase of the study were recruited. They completed questionnaires using the mySCQM patient app and provided two self-collected capillary blood samples following the administration of the 3rd vaccine dose, that were tested for anti-S1-IgG levels. The analysis included patients who received a 3-dose homologous vaccination and reported no SARS-CoV-2 infection between the 2nd and 3rd doses. Mixed effects continuous outcome logistic regression and Cox proportional hazards regression models were employed to address the study aims. We adjusted for potential confounders at the time of the 1st vaccination.
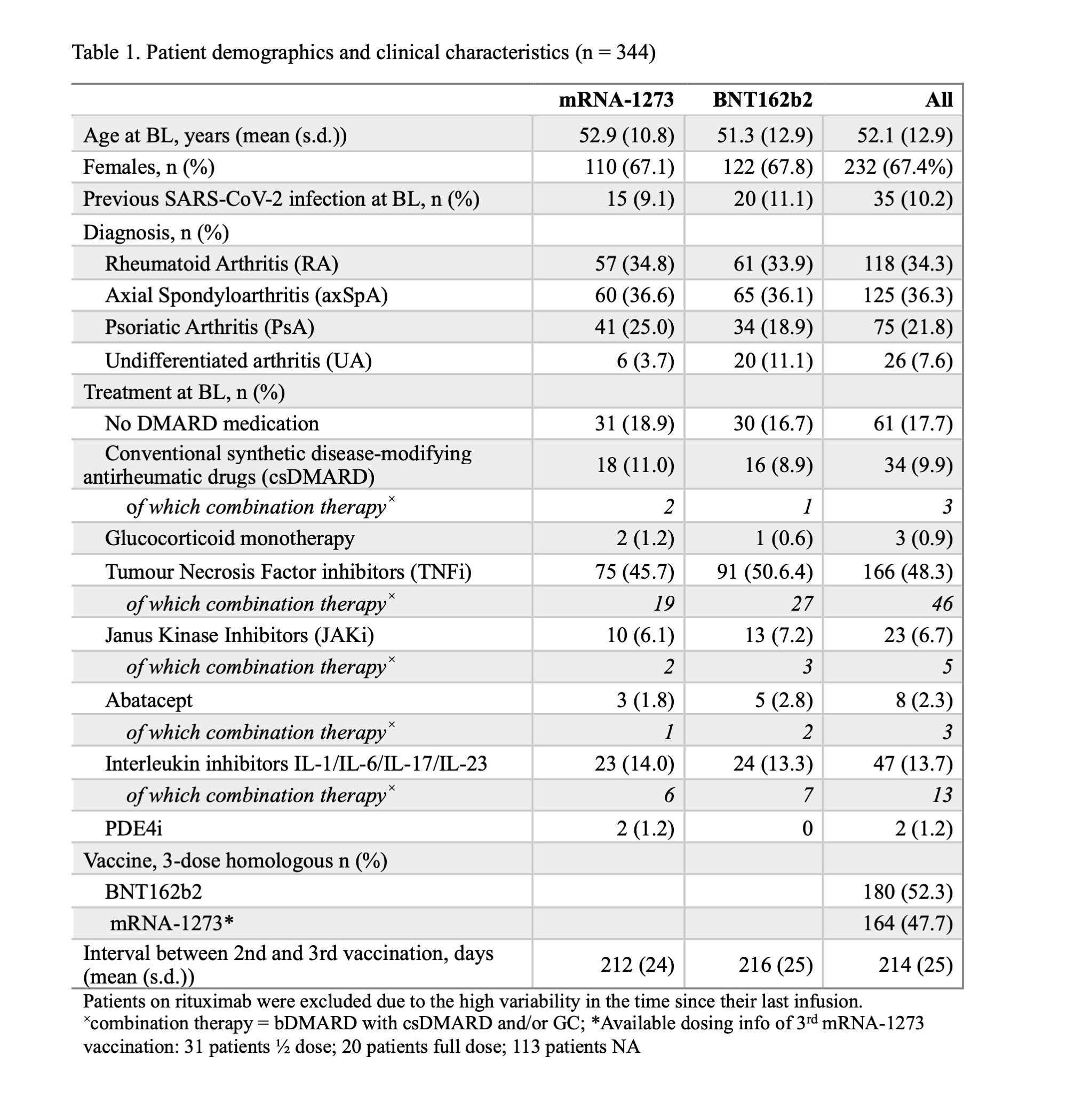
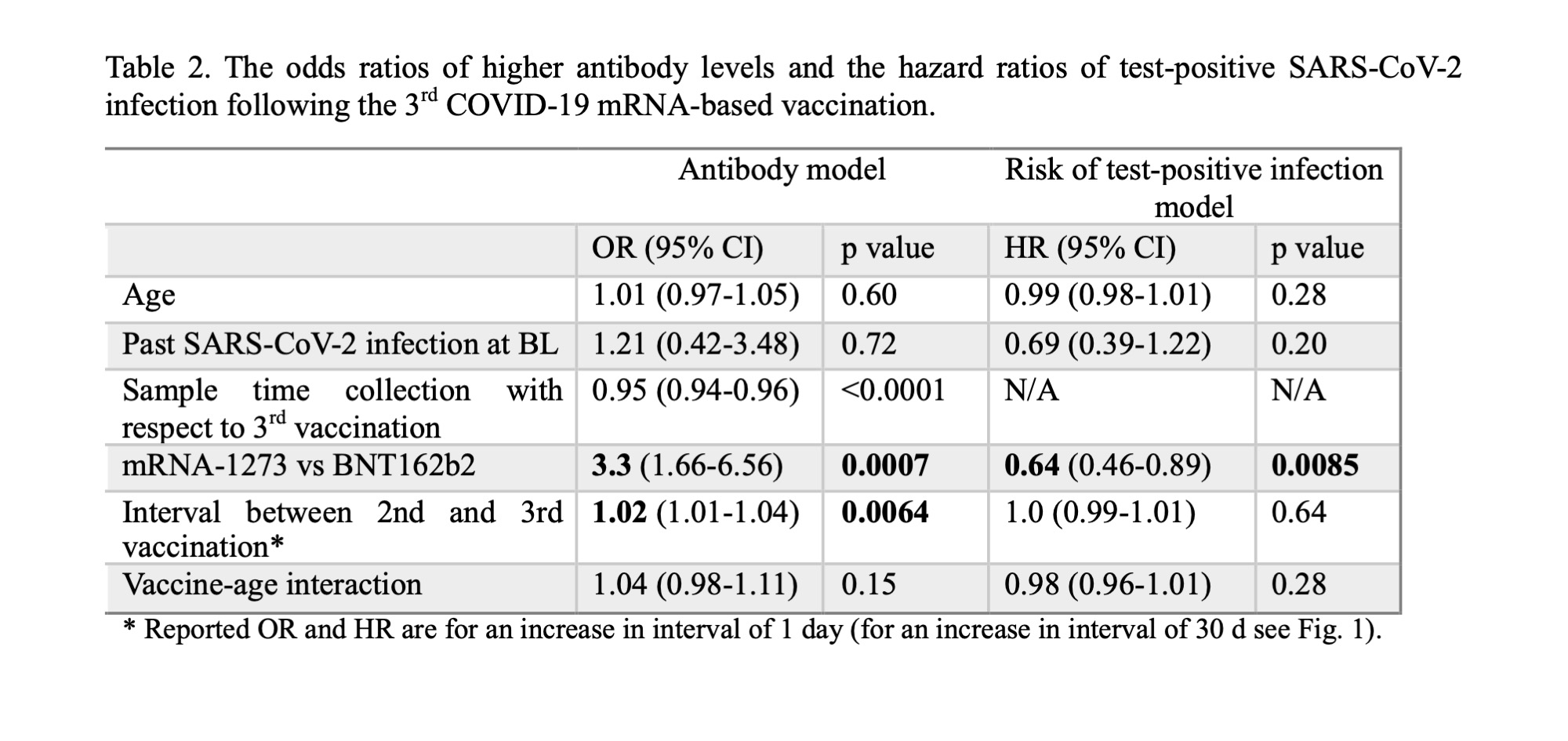
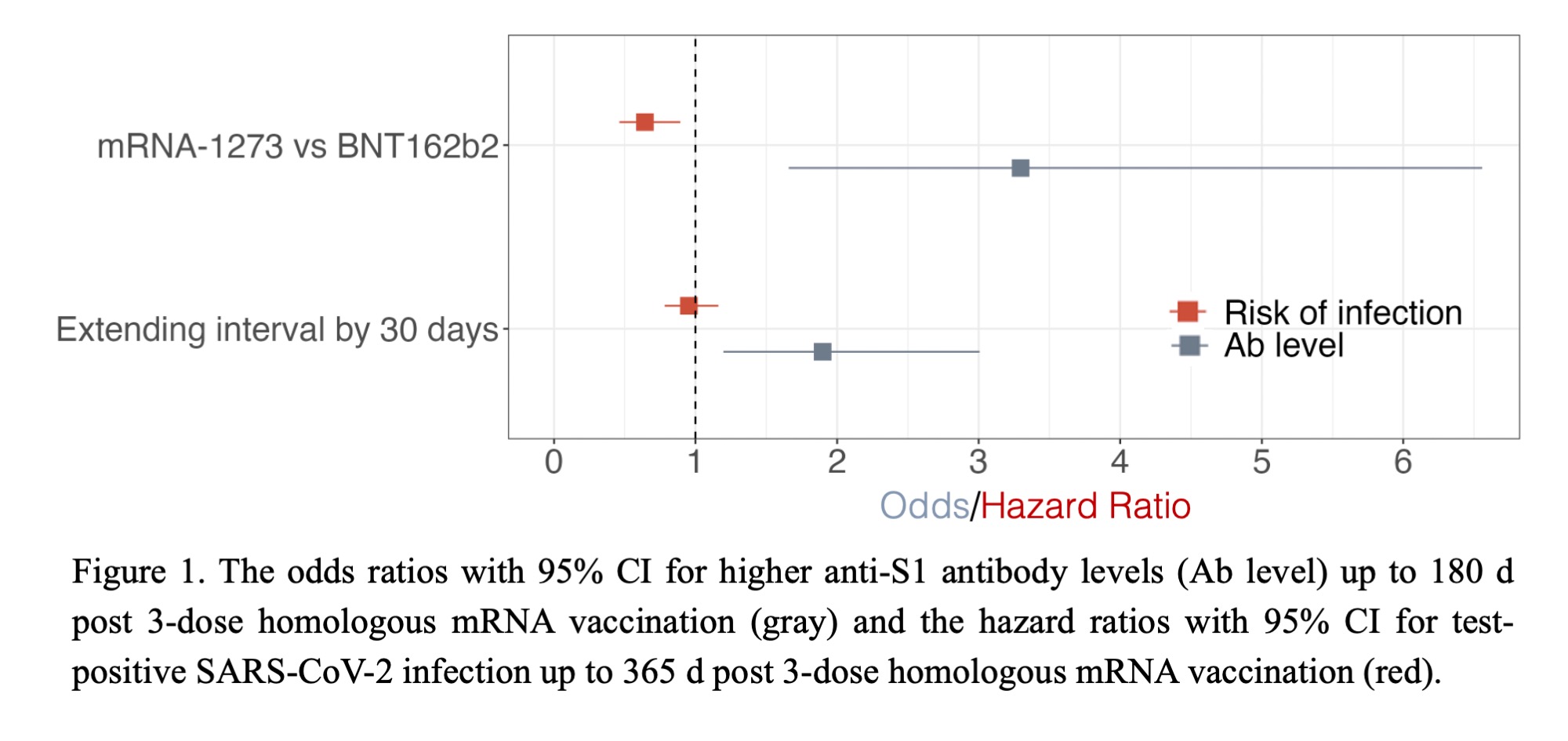
Results: 344 IRD patients (67% female, mean age 52 y, 34% RA, 36% axSpA, 22% PsA, 8% UA) received their 3rd COVID-19 vaccination between 2021-08-13and 2022-03-04. The mean interval between the 2nd and 3rd vaccinations was 214 d (s.d. 25 d). The longer the interval to the booster dose was, the higher the antibody response to the 3rd dose up to 180 d post immunization. This result persisted after adjusting for confounders (Table 2). Indicatively, prolonging the vaccination interval by 30 d, led to a 1.9-fold increase (95% CI 1.2 – 3.0) of the odds of higher antibody levels post 3rd vaccination (Fig. 1).
154 patients reported a SARS-CoV-2 infection after the 3rd vaccine dose (between 2021-12-28and 2022-12-05, during the B.1.1.529 Omicron wave). We found no evidence of the vaccination interval affecting the hazard of test-positive SARS-CoV-2 infection up to 365 d post 3rd vaccination (Table 2).
After adjusting for confounders, the odds of higher anti-S1 levels were 3.3 times greater (95% CI 1.7 – 6.6; p< 0.001) in 3-dose homologous mRNA-1273 recipients compared toBNT162b2 recipients (Table 2, Fig. 1). Moreover, the hazard of a test-positive SARS-CoV-2 infection up to 365 d post 3rd vaccination was 36% lower (HR 0.64; 95% CI 0.46 – 0.89; p < 0.01) in 3-dose homologous mRNA-1273 vs BNT162b2 recipients (Table 2, Fig. 1).
Conclusion: A longer vaccination interval and a 3-dose homologous vaccination with mRNA-1273 were associated with higher anti-SARS-CoV-2 Spike IgG levels post 3rd vaccination in IRD patients. 3-dose homologous vaccination with mRNA-1273 was also associated with a lower risk of a test-positive SARS-CoV-2 infection post 3rd vaccination.
1. X. Zhao et al., N. Engl. J. Med.386, 894–896 (2022).
2. T. A. Bates et al., JCI Insight. 8 (2023)
3. C. E. Raptis et al., Front. Immunol.13, 1–11 (2022).
To cite this abstract in AMA style:
Raptis C, Berger C, Polysopoulos C, Ciurea A, Andrey D, Maletic T, Riek M, Scherer A, von Loga I, Safford J, Lauper K, Moeller B, Vuilleumier N, Finckh A, Rubbert-Roth A. An Extended Interval Between mRNA COVID-19 Booster Vaccinations Is Associated with an Increased Humoral Immune Response in Patients with Inflammatory Rheumatic Diseases [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/an-extended-interval-between-mrna-covid-19-booster-vaccinations-is-associated-with-an-increased-humoral-immune-response-in-patients-with-inflammatory-rheumatic-diseases/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/an-extended-interval-between-mrna-covid-19-booster-vaccinations-is-associated-with-an-increased-humoral-immune-response-in-patients-with-inflammatory-rheumatic-diseases/