Session Information
Date: Saturday, November 16, 2024
Title: Abstracts: Systemic Sclerosis & Related Disorders – Basic Science
Session Type: Abstract Session
Session Time: 1:00PM-2:30PM
Background/Purpose: Interstitial lung disease (ILD) is a major cause of morbidity and mortality in systemic sclerosis (SSc). We aimed to identify circulating immune cells associated with SSc-ILD.
Methods: We utilized single-cell RNA sequencing (39 SSc-ILD and 28 SSc without ILD) and mass cytometry (53 SSc-ILD, 29 SSc without ILD, 18 controls) using cryopreserved PBMC to characterize immune cell subsets associated with SSc-ILD. Additional methods including lung IHC staining, bulk RNA sequencing, and cytotoxicity assays were used to evaluate contributions to lung tissue damage and to elucidate the regulation of disease-relevant cells.
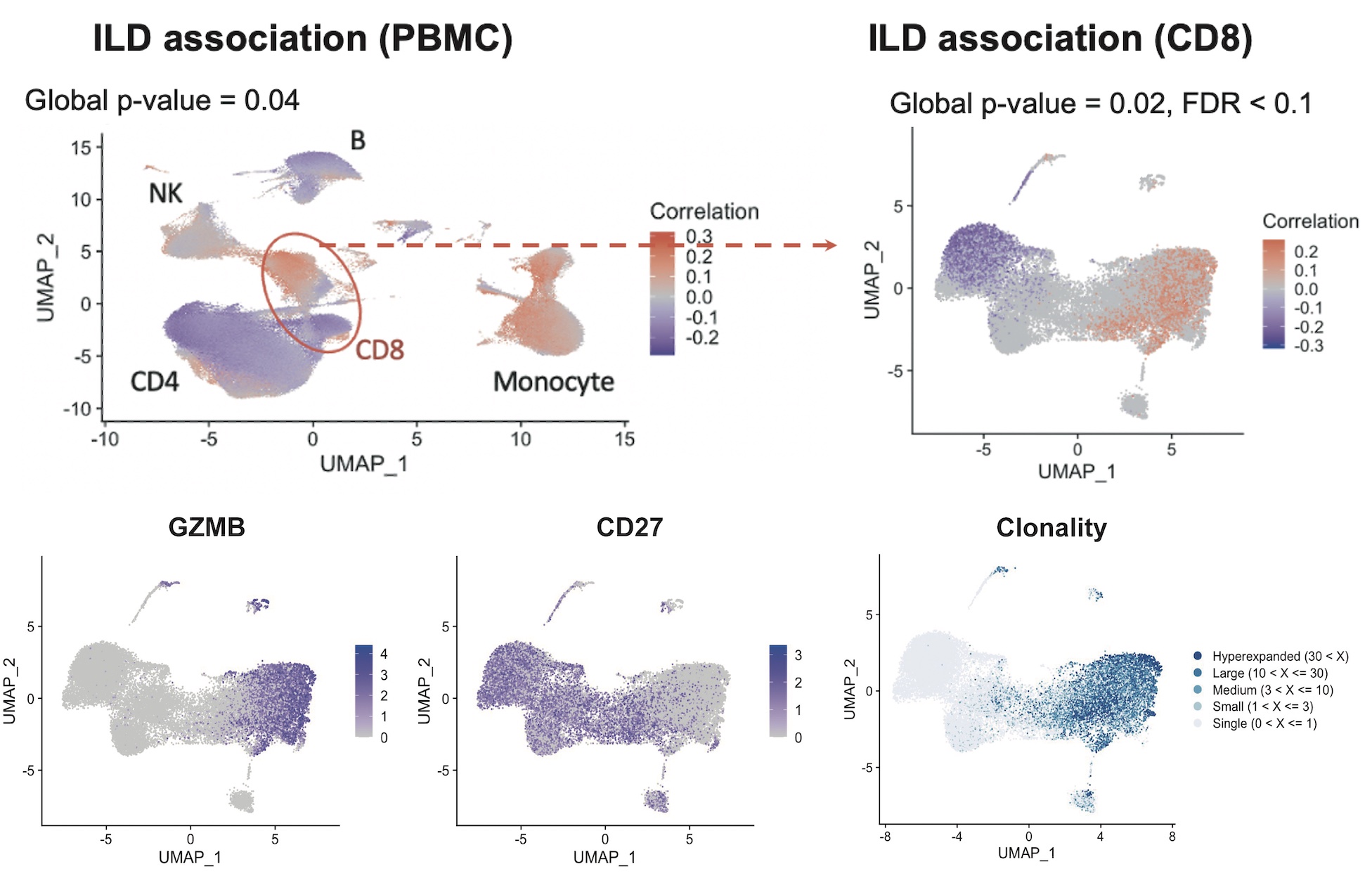
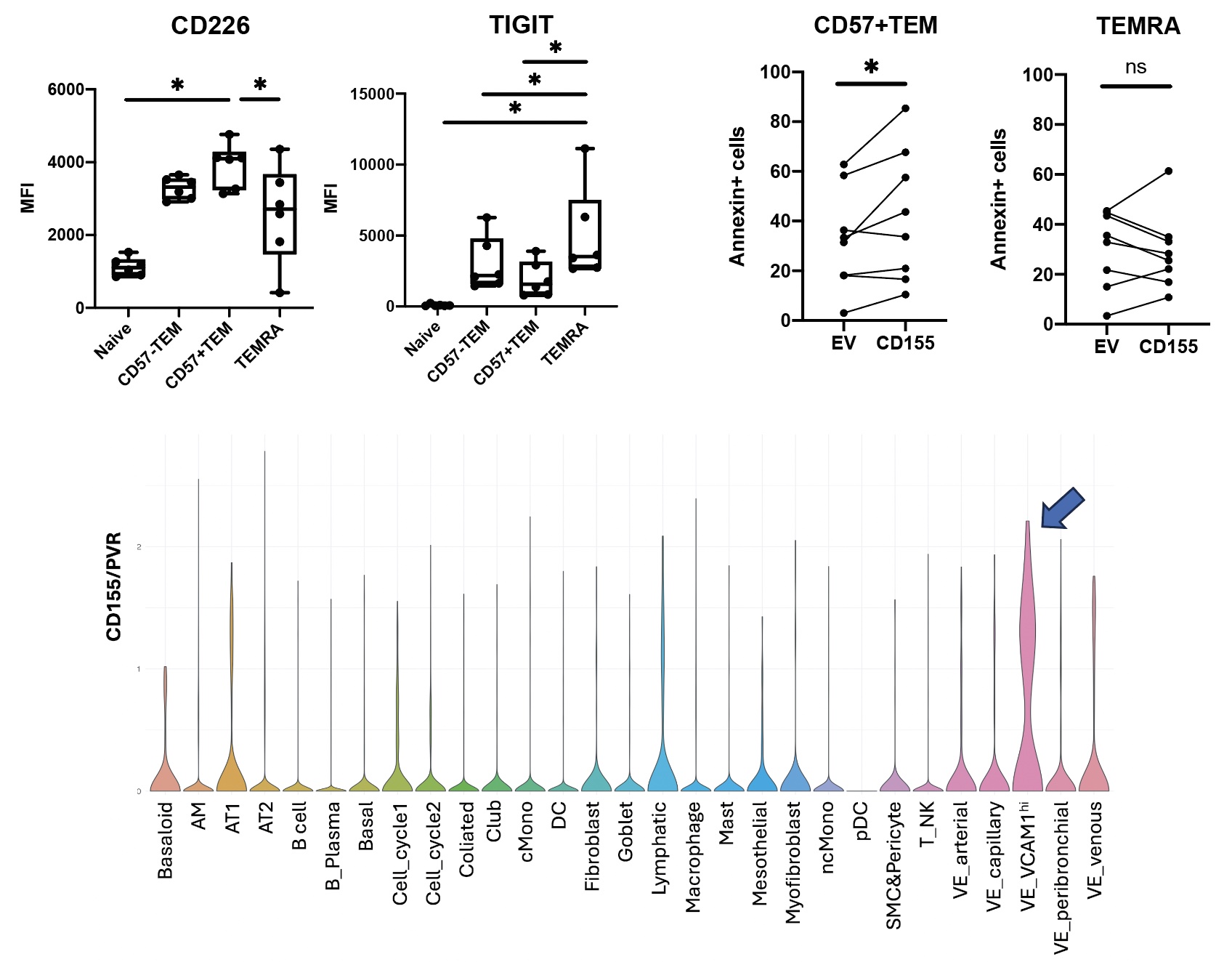
Results: A comprehensive single-cell RNA sequencing analysis of PBMC highlighted that a subset of CD8 T cells was significantly associated with ILD among SSc patients. Among CD8 T cells, a cluster containing CD27- GZMB+ was significantly enriched in patients with SSc-ILD compared to SSc without ILD (Figure 1). TCR repertoire analysis indicated that the cluster was clonally expanded, suggesting antigen-driven expansion. Parallel mass cytometry analysis of the same patient cohorts confirmed that a similar cytotoxic-appearing T cell population, characterized as CD57+ CD27- CD56- CD45RO+ effector memory CD8 T cells (CD57+ TEM), was significantly increased in SSc-ILD patients. IHC staining confirmed that CD57+TEM infiltrates SSc-ILD lung tissues. Bulk RNA sequencing of CD57+ TEM and TEM re-expressing CD45RA (TEMRA) CD8 T cells from 5 SSc-ILD patients revealed that CD57+ TEM had similar levels of GZMA, GZMB, and PRF1 to TERMA. Differential gene expression analysis identified 939 DEGs between CD57+ TEM and TEMRA, including high expression of ITGA4 (integrin α4) and ITGB1 (integrin β1), which constitute VLA4, in CD57+ TEM. In addition, CD226, a costimulatory receptor for CD155/PVR, was highly expressed on CD57+ TEM. Conversely, TIGIT, an inhibitory receptor for CD155/PVR, was highly expressed on TEMRA. Independent flow cytometry analysis confirmed the high expression of CD226 on CD57+ TEM and the high expression of TIGIT on TEMRA. Cytotoxicity assays indicated that CD155/PVR enhances the killing activity of CD57+ TEM but does not affect TEMRA, suggesting that CD155/PVR has a differential impact on the function of CD57+ TEM versus TEMRA. Publicly available single-cell RNA sequencing data from ILD lung tissues indicated that vascular endothelial cells specifically express CD155/PVR (Figure 2). Furthermore, this cluster also showed increased expression of VCAM1, the receptor for VLA4, suggesting that vascular endothelial cells may promote the activation and infiltration of CD57+TEM into the lungs through interactions with CD226 and VLA4.
Conclusion: Our study identified a significant expansion of cytotoxic effector memory CD8 T cells in the circulation of patients with SSc-ILD. These cells are present in affected lungs, and their function may be regulated by CD155 on vascular endothelial cells in SSc-ILD.
A. Expression of CD226 and TIGIT assessed by flow cytometry (SSc-ILD, n=6). B. Cytotoxicity of CD57+TEM and TEMRA with empty vector L cells or CD155-expressing L cells (n=8). C. Expression of CD155/PVR in lung tissue scRNAseq from the ILD study (ref1). VE: Vascular endothelial cells. Ref 1: Am J Respir Crit Care Med 2019 Jun 15;199(12):1517_1536.
To cite this abstract in AMA style:
Sasaki T, Cao Y, Marks K, Ainsworth R, Taylor K, Bottini N, Elahee M, Kamiya M, Kim E, Boin F, Rao D. An Expanded Cytotoxic CD8 T Cell Population Regulated by CD155-CD226 in SSc-ILD [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/an-expanded-cytotoxic-cd8-t-cell-population-regulated-by-cd155-cd226-in-ssc-ild/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/an-expanded-cytotoxic-cd8-t-cell-population-regulated-by-cd155-cd226-in-ssc-ild/