Session Information
Date: Tuesday, November 15, 2016
Title: Systemic Lupus Erythematosus – Clinical Aspects and Treatment - Poster III: Biomarkers and Nephritis
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: The SLE-key® test to Rule Out lupus was developed by ImmunArray Ltd using serum samples of 246 SLE patients and 252 self-declared healthy controls. The test was validated to rule out SLE with 94% sensitivity and 75% specificity and a negative predictive value (NPV) of 93%1. Here we evaluate the performance of the SLE-key® test in aiding the management of suspected lupus patients in a large clinical practice.
Methods: We analyzed the SLE-Key® test results from 55 patients being evaluated and managed for SLE at the Rheumatology and Immunotherapy Center, in Franklin, WI. Serum samples were collected from individual subjects with informed consent and sent for testing at VERACIS (Richmond, VA), using the SLE-Key® iCHIP®2 which contains ~200 antigens and detects profiles of IgG and IgM antibodies. We evaluated the 55 patients clinically using SLICC criteria, and we compared our clinical impression to the SLE-Key® test results. We also evaluated the impact of the test on our subsequent management decisions – terminating evaluation; further evaluation; or initiation of definitive SLE therapy.
Results:
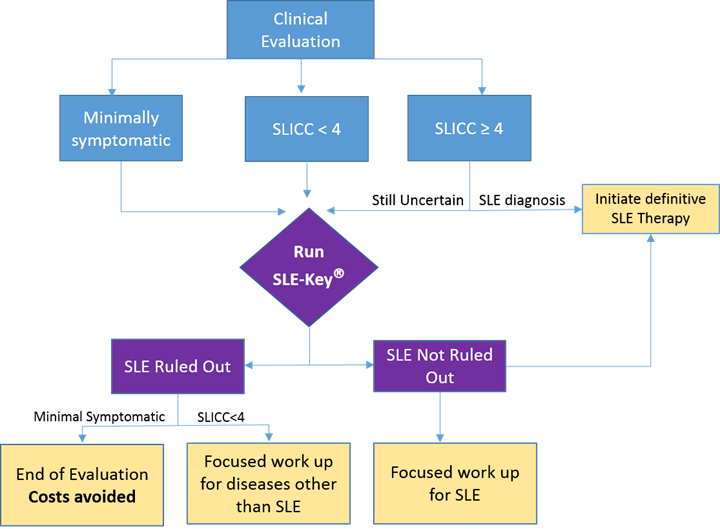
Of the 55 patients tested, 24 were minimally symptomatic, 19 symptomatic patients fulfilled standard SLICC criteria and manifested scores of ≥4, and the remaining 12 patients were symptomatic but did not meet SLICC criteria and manifested a score <4 (Figure 1 shows a flowchart of the study).
The SLE-Key® test confirmed our clinical impression for 30 of the 55 patients (54.5%). SLE was definitively ruled out in 87.5% (21/24) of the minimally symptomatic patients who were assigned to “end of SLE evaluation” with no further follow up or laboratory testing. Of the 19 patients who fulfilled standard SLICC criteria and manifested ACR scores of ≥4, 17 were not ruled out by the SLE-Key® test. In 4 of these cases, clinical impression was revised to ‘SLE’ based on SLE-key® test results leading to accelerated initiation of therapy.
SLE-Key® test results contributed to a change in clinical diagnosis in 10 patients (18%); in 3 of the minimally symptomatic patients, we revised our diagnosis from “SLE” to “Not SLE” and no further evaluation was needed. In the remaining 7 symptomatic cases, (3 of whom manifested SLICC score of <4) we changed our diagnosis to SLE and accelerated time to therapy.
Conclusion: The SLE-key® rule out test provides a laboratory aid to improve the diagnostic and dispositive efficiency of a new clinical paradigm; thus saving undue concern, time and resources both to the patient and to the healthcare system. In 25/55 patients in this cohort, the SLE-key® test provided actionable clinical information, leading to termination of evaluation for SLE or initiation of therapy. Multi-center validation is warranted to further define the clinical advantages of this serologic test.
References:
1Putterman et al., Journal of Immunological Methods, 2016
2Fattal et al; Immunology, 2010
Figure 1:
To cite this abstract in AMA style:
Massenburg D, Oldenberg J, Sell A, Krause T, Wells AF. An Antigen Microarray to Rule-out Systemic Lupus Erythematosus, the SLE-Key® Rule-out Test, Performs Well As an Aid in Clinical Practice [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/an-antigen-microarray-to-rule-out-systemic-lupus-erythematosus-the-sle-key-rule-out-test-performs-well-as-an-aid-in-clinical-practice/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/an-antigen-microarray-to-rule-out-systemic-lupus-erythematosus-the-sle-key-rule-out-test-performs-well-as-an-aid-in-clinical-practice/