Session Information
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Biologic agents are integral to the treatment of juvenile idiopathic arthritis (JIA) and associated uveitis. Pediatric rheumatologists may increase the dosage of biologics beyond the labeled ranges in order to achieve better disease control or decrease the dosage when the disease is well-controlled. Though alternative dosing of biologics in the treatment of JIA and uveitis is often discussed anecdotally, there have been few published studies. We used the Childhood Arthritis and Rheumatology Research Alliance (CARRA) Registry to describe alternative dosing of biologics.
Methods: Patients with JIA enrolled in the CARRA Registry and ever treated with a biologic after enrollment were eligible for the study. We defined high-dose and low-dose as 40% higher or lower than the upper or lower limits of the labeled dose (standard dose), respectively, to exclude instances of minor dose variations due to changes in patients’ body weight or rounding of doses for convenience. When a labeled dose was not available, we determined a standard dose using phase 3 clinical trials and published studies. We assessed the number of patients with use of low- and high-dose biologics (patients could be counted in more than one dose category). We also reported the subset patients who initiated treatment with a biologic for the first time following Registry enrollment and who initiated low-, standard- and high-dose.
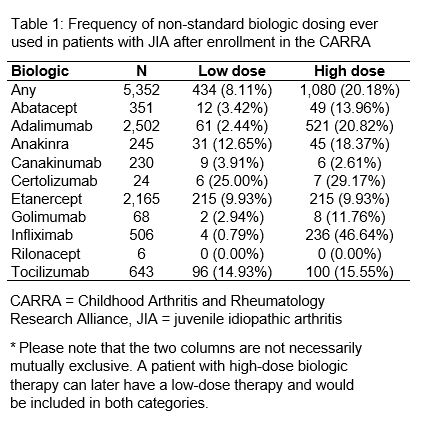
Results: We assessed 5,352 patients treated with 6,740 different biologics following enrollment. At the patient-level, 1,080 (20%) patients ever received high-dose, and 434 (8%) patients ever received low-dose (Table 1). Commonly used biologics that had the highest proportion of patients ever receiving high-dose included infliximab (47%), adalimumab (21%), and anakinra (18%). Commonly prescribed biologics that had the highest proportion of patients ever receiving low-dose included tocilizumab (15%) and anakinra (13%). There were 951 new biologic users after Registry enrollment; 3% were prescribed low-dose, 87% standard-dose, and 11% high-dose.
Conclusion: More than 25% of patients with JIA treated with biologics received doses well outside the standard dose range, despite the fact that there are very little data on the safety and efficacy of such dosing. The vast majority of patients (87%) received standard-dose when initiating their first biologic; therefore, low-dose and high-dose use are generally the result of subsequent dose adjustments rather than disregard for standard dosing. TNF-inhibitors were commonly used at higher doses, especially the monoclonal antibodies that may be used to treat uveitis. Anakinra was commonly used at both high-dose and low-dose, and this may possibly result from variability in individual patient’s responses to anakinra and the relative lack of published data and consensus on recommended dosing. Given the frequent use of low-dose and high-dose biologics in clinical practice, there is an urgent need to assess the relative safety and effectiveness of alternative biologic dosing strategies.
To cite this abstract in AMA style:
Correll C, Shrader P, Dennos A, Phillips T, Shiff N, Verstegen R, Beukelman T. Alternative Dosing of Biologic Therapies Is Frequent Among Children with Juvenile Idiopathic Arthritis in the Childhood Arthritis and Rheumatology Research Alliance Registry [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/alternative-dosing-of-biologic-therapies-is-frequent-among-children-with-juvenile-idiopathic-arthritis-in-the-childhood-arthritis-and-rheumatology-research-alliance-registry/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/alternative-dosing-of-biologic-therapies-is-frequent-among-children-with-juvenile-idiopathic-arthritis-in-the-childhood-arthritis-and-rheumatology-research-alliance-registry/