Session Information
Date: Monday, November 9, 2015
Title: Reproductive Issues in Rheumatic Disorders: Basic and Clinical Aspects
Session Type: ACR Concurrent Abstract Session
Session Time: 4:30PM-6:00PM
Background/Purpose: Pregnancy in patients with SLE is
associated with increased risk of maternal and fetal complications. Studies in
experimental models and humans suggest that complement activation contributes
to fetal loss, preeclampsia and growth restriction. In a case control
longitudinal study of pregnancy from PROMISSE (Predictors of pRegnancy Outcome:
BioMarkers In antiphospholipid antibody Syndrome and Systemic
Lupus Erythematosus),
we investigated whether circulating levels of maternal complement C3 and its
degradation product iC3b would vary during pregnancy and would be associated
with adverse pregnancy outcomes (APO) in SLE patients.
Methods:
The PROMISSE Study enrolled
pregnant women with ≥4 ACR SLE criteria and/or aPL antibodies and healthy
pregnant controls (HC). Patients
were considered aPL positive if aCL and/or antiβ2GP1
were ≥40 IU IgG or IgM and/or LA was
positive in ≥2 determinations with at least once during pregnancy. Exclusion criteria were multi-fetal pregnancy,
prednisone >20 mg/d, proteinuria >1 gm/24hr, and creatinine >1.2
mg/dL. APOs were defined as fetal death, neonatal death, preterm delivery
<36 wks due to preeclampsia or placental insufficiency, and/or growth
restriction <5th %ile. Fifty four SLE patients (18 with APO and 36 without)
and 40 HCs were included in this study. C3 and iC3b were measured once per
trimester in serial samples from maternal plasma using investigational lateral
flow assays (Kypha, Inc. St. Louis, MO). Data were log transformed and analyzed
(GraphPad Prism 6).
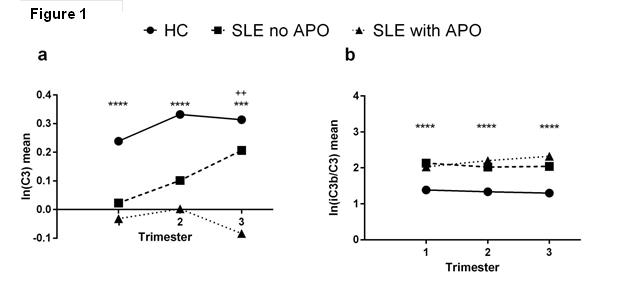
Results: Compared with HC, patients with SLE
had lower C3, higher iC3b levels, and elevated iC3b/C3 ratios throughout pregnancy
(Fig. 1, ****p<0.0001, p<0.001***,
Tukey’s post-hoc ++). Although
neither C3 nor iC3b early in pregnancy predicted APO (Fig. 1b), C3 levels were
lower in the 3rd trimester in SLE with late APOs (n=13) compared
with uncomplicated SLE and HC pregnancies (n=41) (Fig. 1b. ANOVA: F=9.213,
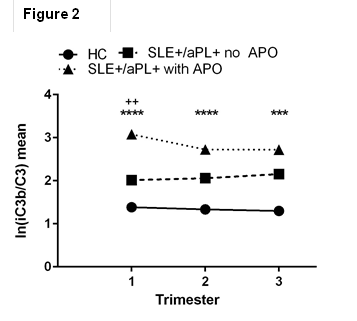
P<0.001, Tukey’s post-hoc). In the subset of aPL-positive SLE patients
(n=13), elevated iC3b/C3 ratios were associated with APOs (n=5) (Fig. 2, ANOVA:
F=19.48, ****p<0.0001, p<0.001***,
Tukey’s post-hoc ++).
Conclusion: During pregnancy, patients with SLE
show evidence of complement activation and consumption. Lack of an increase in
circulating C3 as pregnancy proceeds is associated with late APOs. If this is
confirmed in larger populations accurate and rapid measurements of C3 and iC3b point
of care assays may prove valuable in the management of SLE pregnancies.
To cite this abstract in AMA style:
Guerra MM, Schmidt M, Kaplowitz E, Strand V, Salmon JE. Alterations in Complement C3 and iC3b in SLE Pregnancies [abstract]. Arthritis Rheumatol. 2015; 67 (suppl 10). https://acrabstracts.org/abstract/alterations-in-complement-c3-and-ic3b-in-sle-pregnancies/. Accessed .« Back to 2015 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/alterations-in-complement-c3-and-ic3b-in-sle-pregnancies/