Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
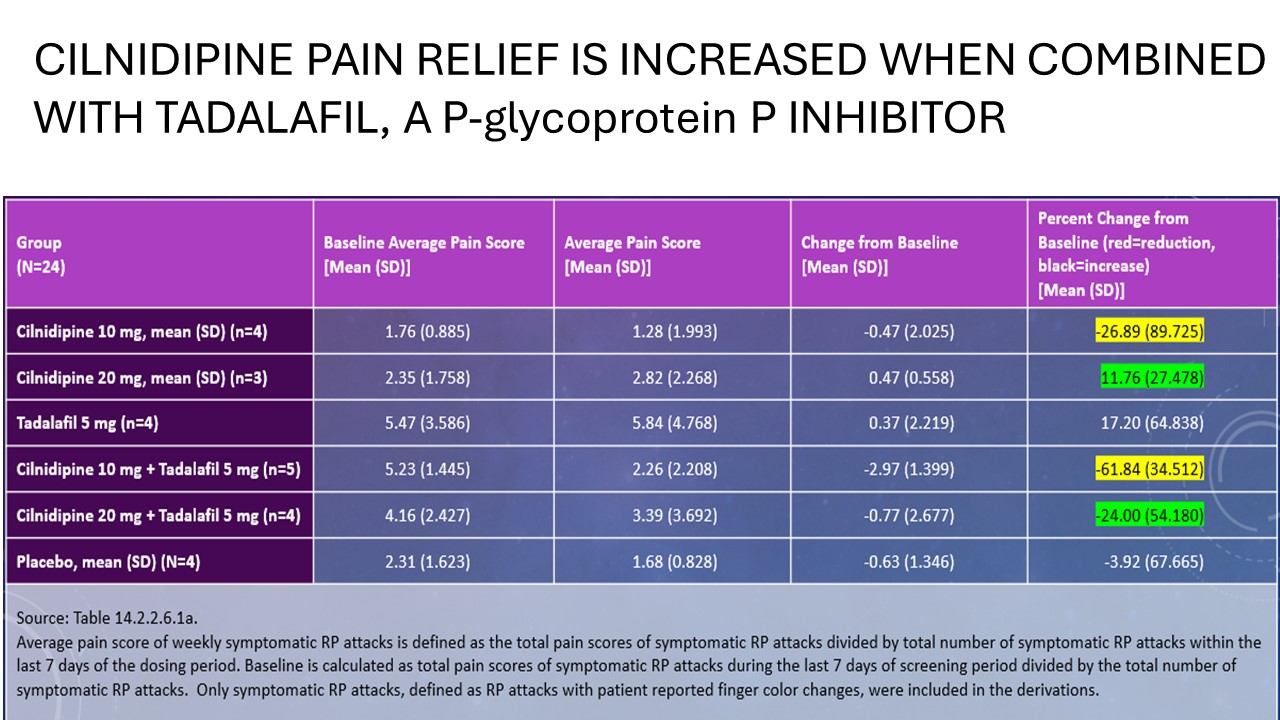
Background/Purpose: Improved treatments for Raynaud’s phenomenon in Systemic Sclerosis (SSc) are needed. A novel oral calcium channel blocker (cilnidipine), with N-type channel antagonism is being developed for Raynaud’s and SSc. An N-type antagonist, ziconotide, is approved for neuropathic pain. Ziconotide has cured erythromelalgia, caused by excessive activity of Nav1.7, a sodium receptor channel. Cilnidipine might similarly block Nav1.7 and Nav1.8, another analgesic target, suggested by SAR analysis. A Phase 2 trial of cilnidipine in Raynaud’s and SSc is ongoing. Patients completed the SHAQ before and after dosing. Pain was markedly reduced after treatment, not driven by Raynaud’s pain impact. Improvement was greatest when tadalafil which binds plasma proteins was co-administered increasing free cilnidipine. Cilnidipine inhibition of Nav 1.7 and Nav 1.8 may account in part for pain relief in SSc.
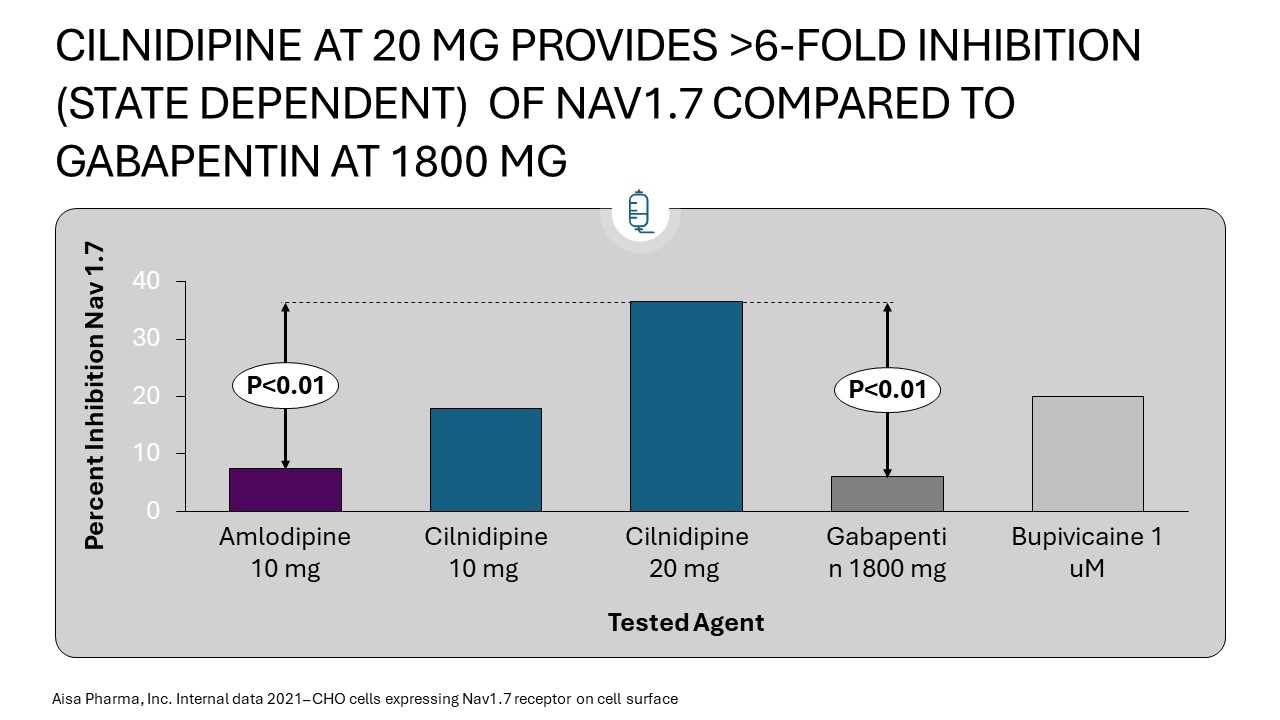
Methods: For Nav 1.7 and Nav 1.8 analyses, CHO cells expressing channels were studied and automated QPatch II® voltage-gated patch clamp performed assays at concentrations of cilnidipine, control, and comparative agents. Experimental results were obtained for closed and inactivated channels. Nav1.7 results were performed before Nav 1.8 effects. Multiple repetitions of the experiment at each drug concentration ensured accuracy and repeatability and IC50 values.
The Phase 2A study, RECONNOITER, is being conducted in Australia. The study has two phases, a dose-finding and initial safety study which is complete and a larger crossover study. The DSMB declared Part A successful after 27 patients were enrolled and analyzed. Part 2 is ongoing. Patients averaged >1 daily Raynaud’s attack to enroll and the results of two weeks of treatment analyzed. Pain scores and other patient-reported metrics were analyzed from the SHAQ.
Results: For Nav 1.7 assays each microarray test at each of 6 concentrations for each agent was performed 54 times. A total of just over 2,100 voltage gated readings were obtained over the course of the experiment. Cilnidipine at the clinical concentration obtained from pk samples in the ongong Phase 2A study was found to more potently inhibit Nav1.7 in the closed state than gabapentin, amlodipine and bupivacaine, at similarly clinically relevant plasma concentrations. Nav 1.8 assessments are in progress.
On the pain domain of the SHAQ, cilnidipine 10 mg(n=4) reduced pain by 27% but 62% with tadalfil 5 mg(n=5) , 20 mg(n=3) increased pain 12% but decreased pain 24% with tadalafil 5 mg(n=4), and placebo patients(n=4) reduced pain 4%.
Conclusion: Cilnidipine seems to have clinically relevant inhibitory properties at Nav 1.7 and may have this at Nav 1.8 as well, based on structure activity relationship analysis and pending experimental determination. In a preliminary Phase 2 study, encouraging effects were seen in pain relief as reported by patients in addition to a reduction in the weekly frequency of Raynaud’s attacks. Clinicians often combine a CCB with a PDE-V inhibitor if response in SSc-RP is inadequate. Addition of a PDE-V may be synergistic when combined with cilnidipine, since free drug concentrations are increased and some central activity may result. Additional data is expected from the ongoing Phase 2 crossover trial.
To cite this abstract in AMA style:
Sternlicht A, Todd M. AISA 021, a Novel Calcium Channel Antagonist in Development for Raynaud’s & Systemic Sclerosis, Has Antagonistic Activity at Sodium Channel Targets for Pain Relief and Treats Scleroderma Pain Better Than Current Calcium Channel Blockers in a Phase 2A Study [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/aisa-021-a-novel-calcium-channel-antagonist-in-development-for-raynauds-systemic-sclerosis-has-antagonistic-activity-at-sodium-channel-targets-for-pain-relief-and-treats-scleroderma-pain-better/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/aisa-021-a-novel-calcium-channel-antagonist-in-development-for-raynauds-systemic-sclerosis-has-antagonistic-activity-at-sodium-channel-targets-for-pain-relief-and-treats-scleroderma-pain-better/