Session Information
Date: Wednesday, October 24, 2018
Title: 6W024 ACR Abstract: SLE–Etiology & Pathogenesis II (2946–2951)
Session Type: ACR Concurrent Abstract Session
Session Time: 11:00AM-12:30PM
Background/Purpose: Systemic lupus erythematosus (SLE) is an autoimmune disorder with a heterogeneous clinical presentation and periods of waxing and waning disease. Heterogeneity in SLE is influenced by genetic and non-genetic susceptibility found in different ethnicities that drive disease expression and severity. The immune pathways that contribute to heightened disease activity in lupus and immune variation by race are critical to understanding SLE disease mechanisms and outcomes.
Methods: Peripheral whole blood samples of European or African American healthy controls (n=18) and SLE patients with either high (SLEDAI≥4) (n=20) or low (SLEDAI<4) (n=20) disease activity were stimulated for 4 minutes with either interferon-a (IFNa), PMA and ionomycin, or Toll-like receptor (TLR) ligands for either TLR4, TLR7/8 or TLR9 for phospho-protein analysis. Phenotype and phospho-protein analysis was assessed by CyTOF and cell heterogeneity was analyzed using t-SNE and manual gating. All SLE patients met ACR classification criteria.
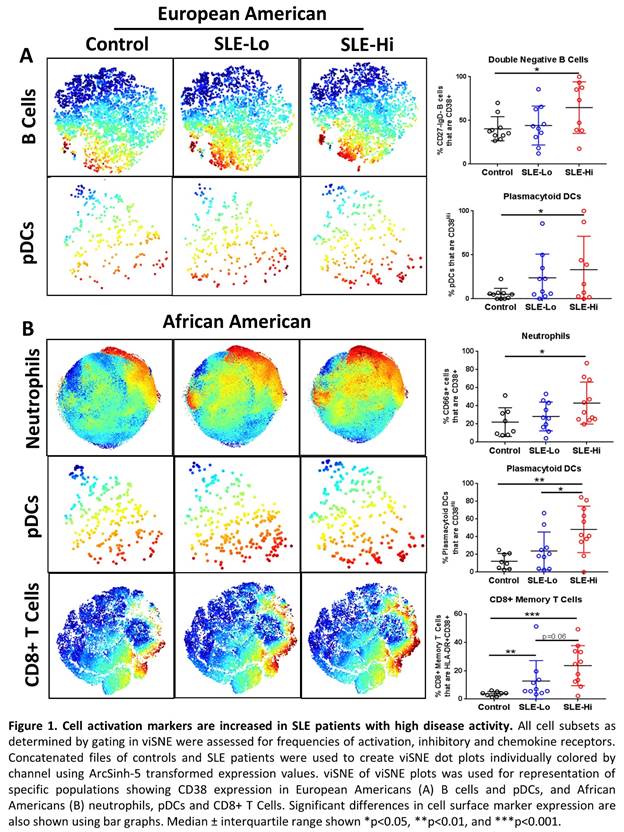
Results: European American SLE patients with high disease activity were differentiated from patients with low disease activity by reduced frequencies of peripheral B cells, specifically naïve B cells (CD27-IgD+CD24lo) (p=0.0101) and double negative B cells (CD27-IgD-) (p=0.0220), while African American patients with high disease activity had elevated frequencies of memory B cells (CD27+IgD-CD38+) (p<0.05) compared to patients with low disease activity. Several cell subsets had increased expression of activation markers during high disease activity including B cells (p=0.0350) and plasmacytoid dendritic cells (pDCs) (p=0.0435) in European Americans (Figure 1A), and neutrophils (p<0.05), pDCs (p=0.005), CD8+ T Cells (p=0.0003) and NKT cells (p=0.0033) in African Americans (Figure 1B). Following whole blood stimulation with IFNa, African American high disease activity patients were distinguished by reduced ability to activate pSTAT5 in almost all major cell populations (p<0.05), and pSTAT3 in monocytes (p=0.0157), granulocytes (p=0.01) and B cells (p=0.0409) compared to low disease activity patients and controls, possibly due to higher basal levels of activation. Further, granulocytes and all antigen presenting cells had reduced activation or unchanged levels of cCASP3, p-p38, pCREB and Syk following TLR7/8 stimulation in African American high versus low disease activity patients (p<0.05). European American high disease activity SLE patients were distinguished by increased signaling in B cells and dendritic cells following TLR4 and TLR7/8 stimulation, specifically in p-p38, pERK1/2, and pCREB (p<0.05).
Conclusion: Our results support a model where race influences heightened SLE disease activity mechanisms with dysregulation in B cell signaling and other antigen presenting cells following TLR7/8 and Type I IFN pathway activation.
To cite this abstract in AMA style:
Slight-Webb S, Smith MC, Maecker HT, Utz PJ, Guthridge JM, James JA. African American and European American SLE Patients with Variable Disease Activity Reveal Distinct Differences in B Cells and TLR7/8 Pathways [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/african-american-and-european-american-sle-patients-with-variable-disease-activity-reveal-distinct-differences-in-b-cells-and-tlr7-8-pathways/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/african-american-and-european-american-sle-patients-with-variable-disease-activity-reveal-distinct-differences-in-b-cells-and-tlr7-8-pathways/