Session Information
Date: Sunday, November 10, 2019
Title: T Cell Biology & Targets in Autoimmune & Inflammatory Disease Poster
Session Type: Poster Session (Sunday)
Session Time: 9:00AM-11:00AM
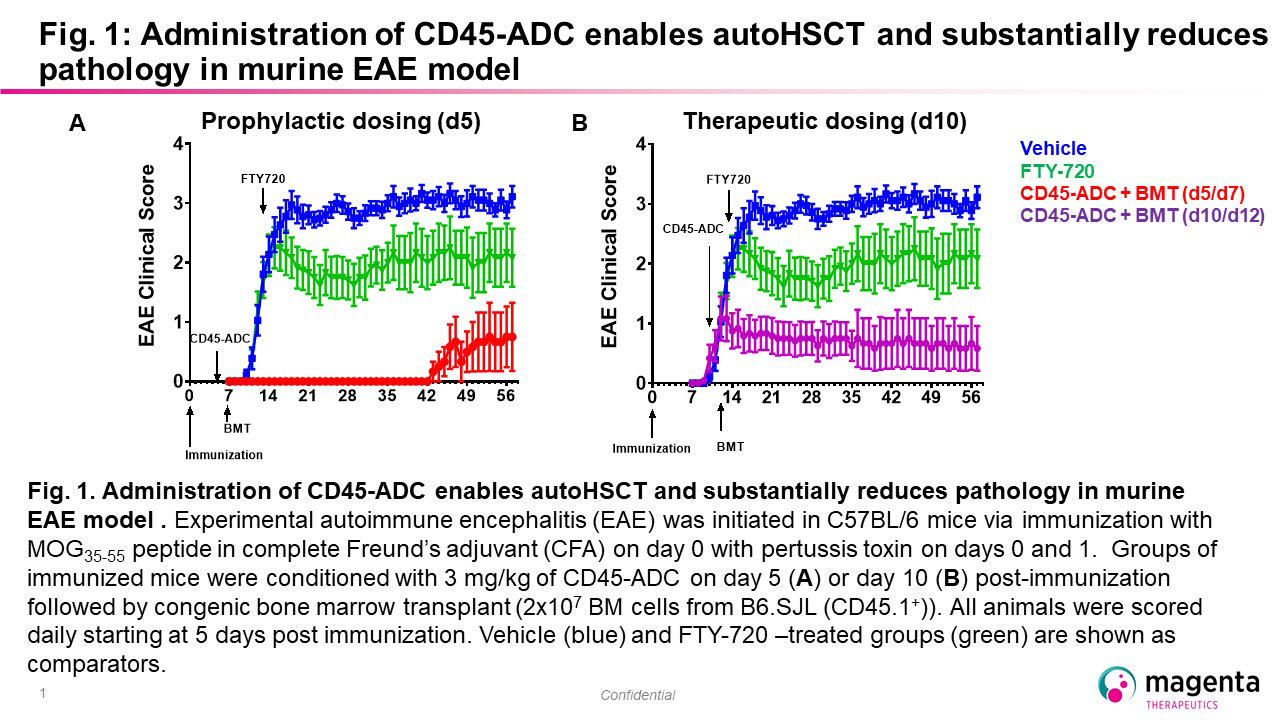
Background/Purpose: Autologous hematopoietic stem cell transplant (autoHSCT) is a highly effective treatment in selected patients with autoimmune diseases. AutoHSCT can induce long-term remission (up to 15 years) with 70-80% progression free survival in patients with relapsed refractory and secondary progressive multiple sclerosis (Muraro 2017) that is superior to standard of care agents in a randomized study (Burt 2019). Likewise, use of autoHSCT in patients with scleroderma achieved superior outcomes in two randomized studies (Tyndall 2014, Sullivan 2018). These impressive results are achieved by eradication of autoreactive immune cells and re-establishment of a self-tolerant immune system, i.e., immune system reset. However, only a small fraction of eligible patients undergo autoHSCT, in part due to toxicity associated with current conditioning protocols. To address these issues, we are developing antibody drug conjugates (ADCs) that selectively target CD45 to eradicate autoimmune cells and enable autoHSCT as a potentially one-time curative treatment for patients with autoimmune disease.
Methods: We generated a novel ADC targeting murine CD45 that was evaluated as a single conditioning agent for murine congenic transplant. This ADC was further evaluated for its ability to eliminate pathogenic host-reactive cells in the context of multiple murine models of autoimmune disease, including MOG-induced experimental autoimmune encephalitis (EAE), proteoglycan-induced arthritis (PGIA), and sclerodermatous graft-vs-host disease (scGVHD). To translate these encouraging pre-clinical data we generated novel anti-human CD45 ADCs that cross react with nonhuman primates (NHP) and evaluated these for the ability to deplete hematopoietic and immune cells in NHPs.
Results: A single-dose of anti-mouse CD45-ADC achieved full myeloablation in recipient mice and enabled full donor chimerism in a congenic mouse transplant model. In EAE, conditioning with anti-mouse CD45-ADC followed by congenic transplant prior to disease onset significantly delayed disease onset and reduced overall disease severity. In active EAE, treatment with anti-mouse CD45-ADC followed by congenic transplant halted progression of disease activity. The disease modifying effect in EAE with anti-mouse CD45-ADC showed comparable efficacy to a traditional conditioning regimen. Evaluation of CD45-ADC in additional autoimmune models of arthritis and scleroderma are ongoing and will be presented. Given these encouraging results we developed an anti-human CD45 ADC that cross-reacts with non-human primates (NHP). Substantial depletion of both lymphocytes and hematopoietic stem cells (HSCs) was observed at well-tolerated doses.
Conclusion: These results suggest that targeted immune depletion with a single treatment of CD45-ADC may be sufficient for auto-HSCT and allow re-establishment of immune tolerance. Targeted CD45-ADCs may represent a safer and better tolerated approach for conditioning patients prior to immune reset through autoHSCT and significantly reduce the side effects associated with current conditioning.
To cite this abstract in AMA style:
Gillard G, Proctor J, Brooks M, Lamothe T, Hyzy S, McDonough S, Palchaudhuri R, Bhat A, Sarma G, Bhattarai P, Sawant P, Pearse B, McDonagh C, Boitano A, Cooke M. Administration of a CD45 Antibody Drug Conjugate as a Novel, Targeted Approach to Achieve Immune System Reset: A Single Dose of CD45-targeted ADC Safely Conditions for Autologous Transplant and Ameliorates Disease in Multiple Models of Autoimmune Disease [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/administration-of-a-cd45-antibody-drug-conjugate-as-a-novel-targeted-approach-to-achieve-immune-system-reset-a-single-dose-of-cd45-targeted-adc-safely-conditions-for-autologous-transplant-and-amelio/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/administration-of-a-cd45-antibody-drug-conjugate-as-a-novel-targeted-approach-to-achieve-immune-system-reset-a-single-dose-of-cd45-targeted-adc-safely-conditions-for-autologous-transplant-and-amelio/