Session Information
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Filgotinib (FIL)—an oral, selective Janus kinase 1 inhibitor (JAKi)—improved RA signs and symptoms in three phase 3 trials. Despite the efficacy of FIL and other JAKi, there are some concerns regarding potential for venous thromboembolism (VTE); major adverse cardiac events (MACE); and elevated platelet levels, which may result from JAK2 inhibition and modulation of thrombopoietin and could be causally related to VTE.
Methods: Patients (pts) meeting 2010 ACR/EULAR criteria for RA who participated in the phase 2 DARWIN 1–2 (D1–2), phase 3 FINCH 1–3 (F1–3), and long-term extension studies (DARWIN 3 [D3] and FINCH 4 [F4]) and received FIL 100 mg, FIL 200 mg, adalimumab (ADA), or placebo (PBO) with or without MTX were included. Events were analyzed as-exposed according to treatment received.
The week (W)12 PBO-controlled safety analysis included data from pts who received FIL 100, FIL 200, or PBO for ≤12W (D1–2, F1–2); some PBO-treated pts had additional data through W24 (F1–2). The active-controlled analysis included data from pts who received FIL 100, FIL 200, or ADA with background MTX (F1) and pts who received FIL 100 + MTX, FIL 200 + MTX, FIL 200, or MTX (F3) for ≤52W. The FIL 200 vs FIL 100 analysis included data from pts (D1–3, F1–4) for ≤5.5 years.
Data were analyzed based on whether pts did/did not experience a MACE or VTE event. For active-controlled analysis sets (except MACE in the MTX-controlled analysis set), exposure-adjusted incidence rates (EAIRs) and 95% confidence intervals (CIs) were calculated using the exact Poisson method, and treatment differences were provided with corresponding CIs based on the confidence limits of individual point estimates. For FIL 200 vs FIL 100 analyses and MACE in the MTX-controlled analysis set, EAIRs, difference in EAIRs, and 95% CIs were calculated using Poisson regression with treatment group as a covariate. Suspected MACE (cardiovascular [CV] death, myocardial infarction, and stroke) and VTE (deep vein thrombosis and pulmonary embolism) were adjudicated by a blinded independent CV safety endpoint adjudication committee.
Results: CV risk factors were common at baseline (Table 1). No VTE occurred in the 12W PBO-controlled safety analysis (Figure 1). There were 13 total VTE events. EAIR/100 patient-years of exposure (PYE) were comparable for all treatments (EAIR [n]: FIL 200, 0.2 [7]; FIL 100, 0.1 [1]; ADA, 0.3 [1]; MTX, 0.5 [2]; PBO (up to W24), 0.7 [2]; Figure 1). Crude incidence of MACE in the PBO-controlled safety analysis is shown in Figure 2. There were 32 MACE events overall. EAIRs were comparable for all treatments (EAIR [n]: FIL 200, 0.6 [16]; FIL 100, 0.6 [10]; ADA, 0.3 [1]; MTX, 0.5 [2]; PBO (up to W24), 1.0 [3]; Figure 2). Platelet counts did not increase during the study; at W52, mean platelet counts slightly decreased in all treatment arms.
Conclusion: No safety signal for VTE or MACE was observed in the filgotinib RA program. EAIRs of positively adjudicated VTE and MACE were low and consistent with expectations in patients with RA (EAIR 0.4/100 PYE for VTE and 1.0/100 PYE for MACE).1,2
References
- Davies R et al. Ann Rheum Dis. 2011;70:1831–4.
- Cooksey R et al. Semin Arthritis Rheum. 2018;48:367–73.
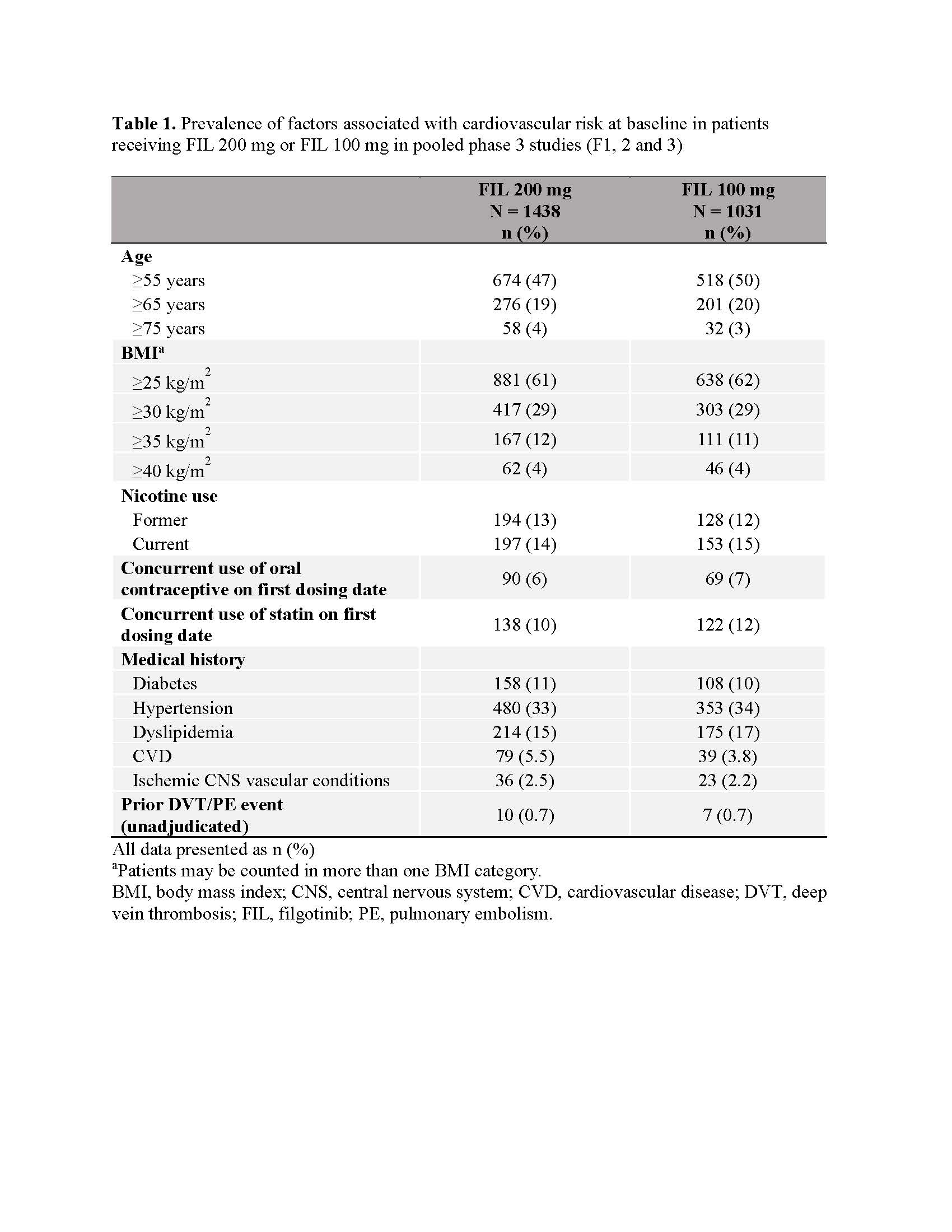 Table 1. Prevalence of factors associated with cardiovascular risk at baseline in patients receiving FIL 200 mg or FIL 100 mg in pooled phase 3 studies (F1, 2 and 3)
Table 1. Prevalence of factors associated with cardiovascular risk at baseline in patients receiving FIL 200 mg or FIL 100 mg in pooled phase 3 studies (F1, 2 and 3)
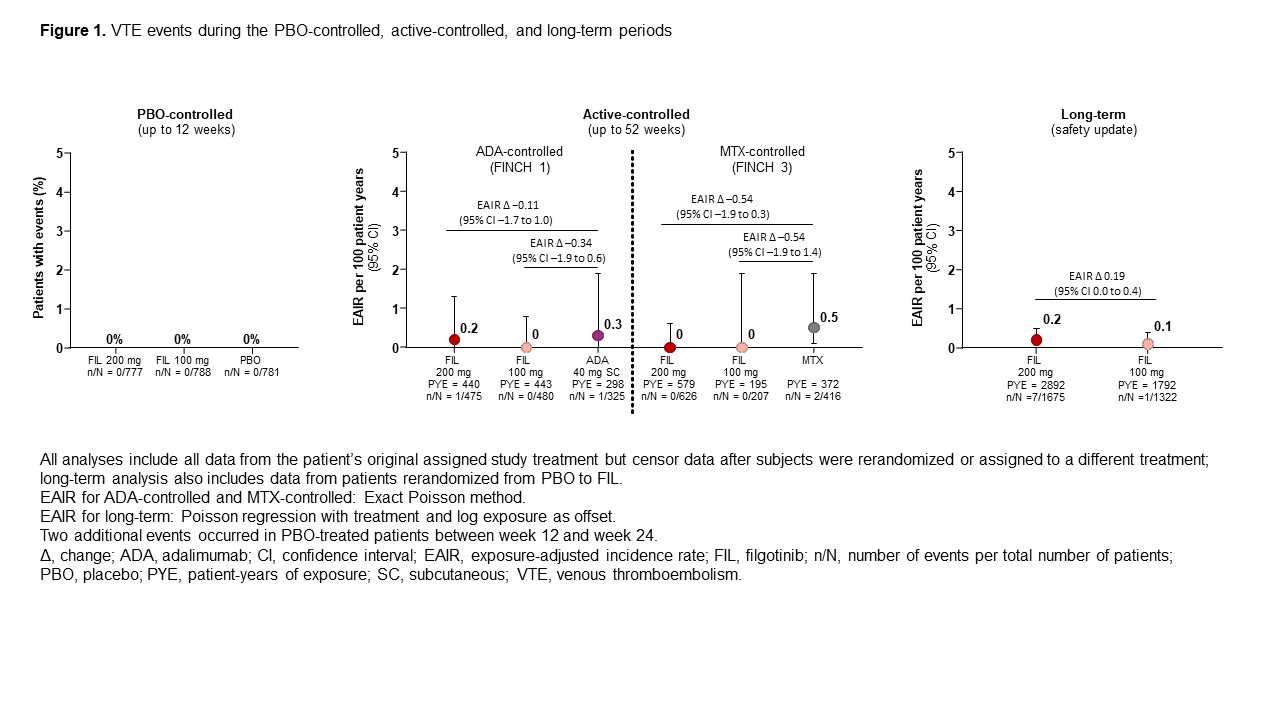 Figure 1. VTE events during the PBO-controlled, active-controlled, and long-term periods
Figure 1. VTE events during the PBO-controlled, active-controlled, and long-term periods
 Figure 2. MACE during the PBO-controlled, active-controlled, and long-term periods
Figure 2. MACE during the PBO-controlled, active-controlled, and long-term periods
To cite this abstract in AMA style:
Charles-Schoeman C, Bae S, Chopra A, Cohen S, Curtis J, Gottenberg J, Keystone E, Yamaoka K, Nash P, Simon-Campos J, Stohl W, Weinblatt M, Westhovens R, Siegel J, Tiamiyu I, Ye L, Jiang D, Matzkies F, Jahreis A, Sundy J, Giles J. Adjudicated MACE and VTE in the Filgotinib RA Program: Integrated Analysis from Phase 2 and 3 Clinical Trials [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/adjudicated-mace-and-vte-in-the-filgotinib-ra-program-integrated-analysis-from-phase-2-and-3-clinical-trials/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/adjudicated-mace-and-vte-in-the-filgotinib-ra-program-integrated-analysis-from-phase-2-and-3-clinical-trials/
