Session Information
Session Type: Poster Session D
Session Time: 9:00AM-11:00AM
Background/Purpose: Hydroxychloroquine (HCQ) is a commonly prescribed medication for systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), and other rheumatic diseases. HCQ may rarely cause retinopathy. This risk correlates with both daily dose and duration of use1. In addition to screening ophthalmologic examinations, the most recent guidelines by the American Academy of Ophthalmology recommend limiting the dose of HCQ to 5mg/kg/day of actual body weight1. After reviewing our practice’s dosing of HCQ and adherence to these guidelines in 2018, we assessed the impact of sharing prescribing data with individual providers and implementing nurse-aided decision support for HCQ refill requests.
Methods: We previously performed a single-center retrospective analysis of 801 adult patients receiving HCQ for SLE or RA in our rheumatology practice at Beth Israel Deaconess Medical Center between January 1, 2018 and December 31, 2018. We calculated the weight-based dose of HCQ at two separate time points, separated by at least 6 months to allow for “loading dose” adjustments. In mid-2019, we implemented a two-pronged quality improvement intervention:
- We shared aggregate dosing averages with all providers in the practice, then privately shared individual prescribing data with each provider, including a list of patients dosed >5mg/kg/day at both recorded time points in 2018.
- We introduced nurse-aided decision support for HCQ refill requests. For each request, nurses calculated the weight-based dose before sending the request to the prescribing physician along with an alert if the dose was >5mg/kg/day.
One year after the initial analysis, we reviewed the same 801 patients included in the initial analysis, and recorded the current weight and HCQ dose. We used this data to calculate aggregate dosing averages for the practice, and dosing changes for individual patients. We used McNemar’s test to assess for statistical significance of the changes.
Results: Of 801 patients included in the 2018 analysis, 674 continued to receive their care within our practice and remained on HCQ through the end of 2019. 154 patients were dosed >5mg/kg/day at both measured time points during 2018, and of those patients, 93 (60%) were dose-reduced to £5mg/kg/day by date 3. Between Date 2 in 2018 (“pre-intervention”) and Date 3 in 2019 (“post-intervention”), there was a statistically significant increase in the proportion of patients dosed < 5mg/kg/day, from 74% to 87% (p< 0.0001) (Figure 1). HCQ was discontinued in 232 patients between 2018 and 2019. Of those patients, the most commonly cited reasons were “loss to follow up/moved away” (31%), “not effective” (17%), and “no longer needed” (14%) (Figure 2). In 9 patients (4%), HCQ was discontinued due to possible or confirmed retinopathy identified by ophthalmologic screening exam.
Conclusion: Our quality improvement intervention led to a statistically significant increase in the proportion of patients taking HCQ dosed according to the current recommended guidelines. These findings highlight provider feedback and nurse-aided decision support as two potentially effective strategies for improving adherence with the guidelines.
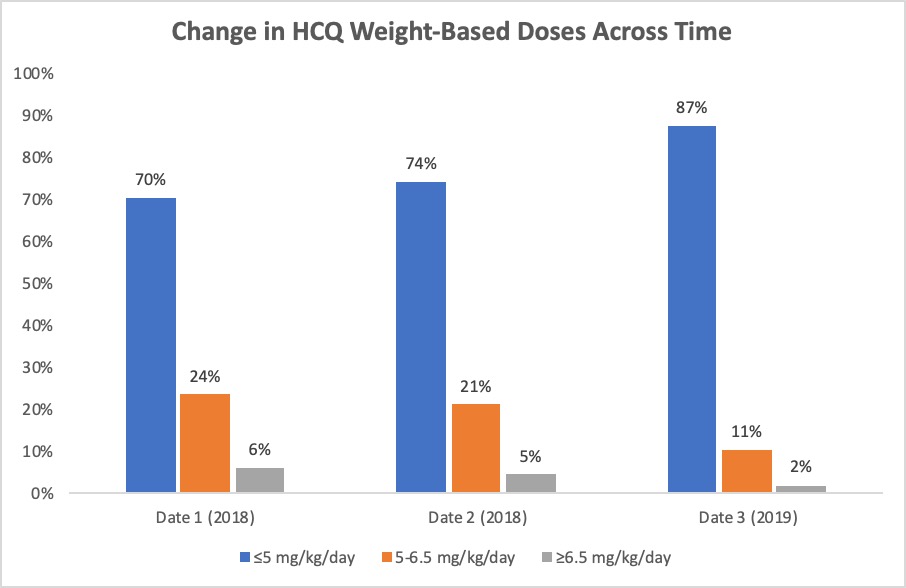 Figure 1: Change in HCQ Weight Based Doses Across Time. Date 1 and Date 2 represent the “pre-intervention” time points in 2018. Date 3 represents the “post-intervention” time point in 2019.
Figure 1: Change in HCQ Weight Based Doses Across Time. Date 1 and Date 2 represent the “pre-intervention” time points in 2018. Date 3 represents the “post-intervention” time point in 2019.
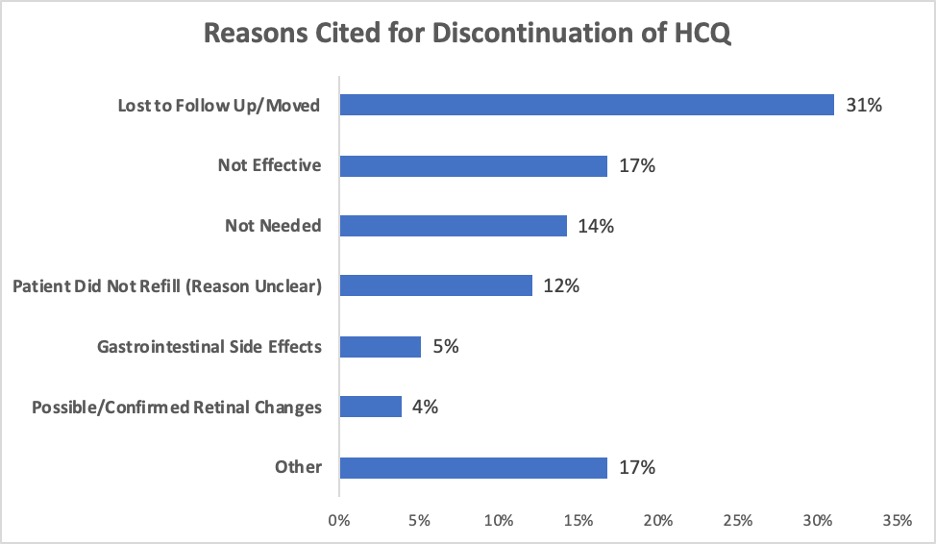 Figure 2: Reasons Cited for Discontinuation of HCQ. “Other” less common reasons for discontinuation included: non-ophthalmologic neurologic side effect, medication interaction, rash, rethinking diagnosis, death of patient, pregnancy, macular degeneration (concern for inability to safely monitor for retinal toxicity), hair loss, hyponatremia, G6PD deficiency, pill size, polypharmacy, tongue swelling, and mood.
Figure 2: Reasons Cited for Discontinuation of HCQ. “Other” less common reasons for discontinuation included: non-ophthalmologic neurologic side effect, medication interaction, rash, rethinking diagnosis, death of patient, pregnancy, macular degeneration (concern for inability to safely monitor for retinal toxicity), hair loss, hyponatremia, G6PD deficiency, pill size, polypharmacy, tongue swelling, and mood.
To cite this abstract in AMA style:
Skorupa T, Shmerling R. Adherence to Weight-Based Dosing Guidelines in Patients Receiving Hydroxychloroquine for Rheumatoid Arthritis and Systemic Lupus Erythematosus: Results of a Quality Improvement Initiative [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/adherence-to-weight-based-dosing-guidelines-in-patients-receiving-hydroxychloroquine-for-rheumatoid-arthritis-and-systemic-lupus-erythematosus-results-of-a-quality-improvement-initiative/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/adherence-to-weight-based-dosing-guidelines-in-patients-receiving-hydroxychloroquine-for-rheumatoid-arthritis-and-systemic-lupus-erythematosus-results-of-a-quality-improvement-initiative/
