Session Information
Date: Sunday, November 13, 2022
Title: RA – Treatment Poster III
Session Type: Poster Session C
Session Time: 1:00PM-3:00PM
Background/Purpose: Patients with IMID, and notably patients with rheumatoid arthritis (RA), are at increased risk of major adverse cardiovascular event (MACE) compared with the general population. It is hence paramount to assess the impact of biological or targeted DMARD (e.g.., tofacitinib and TNFi) on the risk of acute cardiovascular events in patients already at risk, particularly in the context of ORAL Surveillance which showed a higher risk for MACE with tofacitinib, in comparison with TNFI, in RA patients.
Methods: The RELATION study is a retrospective observational cohort study using the French nationwide healthcare database (SNDS). Patients aged 18 years or older, affiliated to the French national health insurance with a diagnosis of RA and initiating tofacitinib after November 1, 2017 or a TNFi after January 1, 2010 (including adalimumab and etanercept, Other TNFi, without previous exposure to tofacitinib) were followed from treatment initiation to December 31, 2020. Patients with a previous history of MACE in the 4 years preceding cohort entry were excluded. All MACE excluding cardiovascular (CV) death were defined by the first hospitalization for MACE using ICD-10 codes or medical procedure code during follow-up. Comorbidities and traditional CV risk factors were identified using hospitalizations, procedures, or medication dispensing in the 4 years prior cohort entry. The unadjusted incidence rate (IR) of MACE excluding CV death was assessed in patients initiating either tofacitinib or a TNFi. The associated 95% confidence intervals (95% CI) were calculated using the exact Poisson distribution and were two-sided.
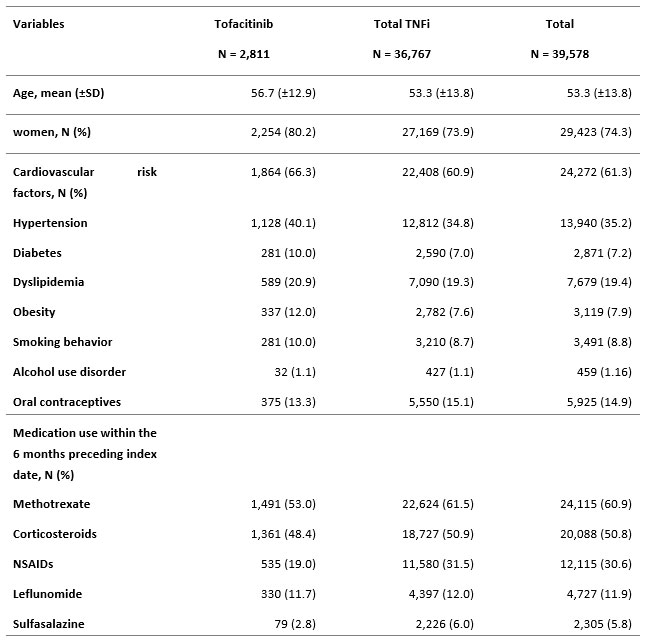
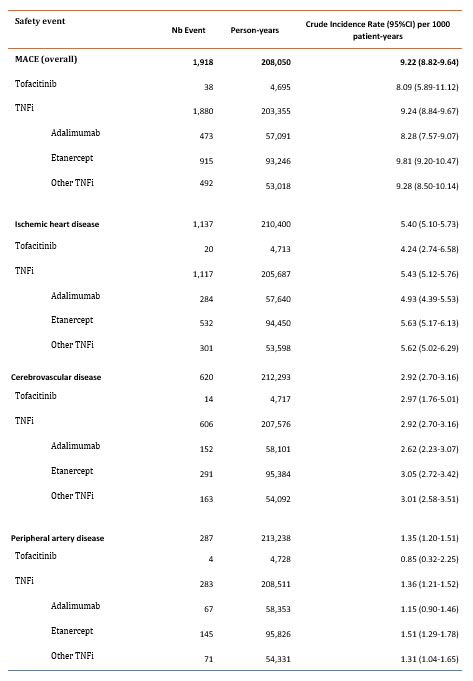
Results: Between 2010 and 2020, a total of 39,578 patients with RA were included. Among these, 2,811 initiated tofacitinib and 36,767 initiated a TNFi (adalimumab: 10,621, Etanercept: 16,512, other TNFi: 9,634). Patients had a mean age of 53 years at cohort entry and were mainly women (72% to 81%). Around 61% of the cohort had at least one CV risk factor, arterial hypertension being the most frequent (35.2%, of the patients) (Table 1). The proportion of patient with CV risk factor was numerically higher in the tofacitinib group. The two major co-medications at treatment initiation in the two groups were methotrexate (60.9%) and corticosteroids (50.8%) (Table 1). 38 incident MACE occurred in the tofacitinib group (IR: 8.1 (5.9-11.1) per 1,000 patient-years (PY)) and 1,880 occurred in the TNFi group (IR: 9.2 (8.4-9.7) per 1,000 PY). The IR per 1000 PY of ischemic heart disease, peripheral artery disease and cerebrovascular disease were respectively 4.24 (2.74-6.58), 0.85 (0.32-2.25) and 2.97 (1.76-5.01) in the tofacitinib group and 5.43 (5.12-5.76), 1.36 (1.21-1.52) and 2.92 (2.70-3.16) in the TNFi group (Table 2).
Conclusion: This study assessed MACE occurrence in patients with RA initiating tofacitinib or TNFi in a French nationwide setting. Adjusted comparative results will allow to compare these occurrences between the different groups of interest.
To cite this abstract in AMA style:
gottenberg j, Mammar N, Kessouri M, RUDANT J, Assi n, grenier B, kirchgesner j. Acute Cardiovascular Events Risk in Rheumatoid Arthritis Patients Treated with Tofacitinib or TNF Inhibitors, a Nationwide Cohort Study: RELATION Study [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/acute-cardiovascular-events-risk-in-rheumatoid-arthritis-patients-treated-with-tofacitinib-or-tnf-inhibitors-a-nationwide-cohort-study-relation-study/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/acute-cardiovascular-events-risk-in-rheumatoid-arthritis-patients-treated-with-tofacitinib-or-tnf-inhibitors-a-nationwide-cohort-study-relation-study/