Session Information
Date: Monday, November 18, 2024
Title: Plenary III
Session Type: Plenary Session
Session Time: 9:00AM-10:30AM
Background/Purpose: This study investigated the diminished expression of Piezo1 (Piezo-type mechanosensitive ion channel component 1), a mechanosensitive receptor, in bone tissues of both patients and mouse models afflicted with glucocorticoid-induced osteoporosis (GIOP). The objective was to delineate the contribution and underlying mechanisms of Piezo1 in GIOP’s pathophysiology and to explore the potential ameliorative effects of activating the Piezo1 pathway.
Methods: Pathophysiological analyses were performed on femoral neck cortical bone samples from GIOP patients and non-GIOP. A GIOP mouse model was established via subcutaneous dexamethasone (DEX) administration (1 mg/kg, five times weekly), and mechanical loading stress was applied to the tibiae (-13N, 0.1s, 40 cycles twice weekly) . The therapeutic efficacy of Yoda1 (a Piezo1 activator, administered at 5 µmol/kg intraperitoneally, five times weekly) was evaluated. Cortical bone RNA sequencing from both human and mouse GIOP/non-GIOP models and in vitro validations using osteocyte-like MLO-Y4 cells were conducted.
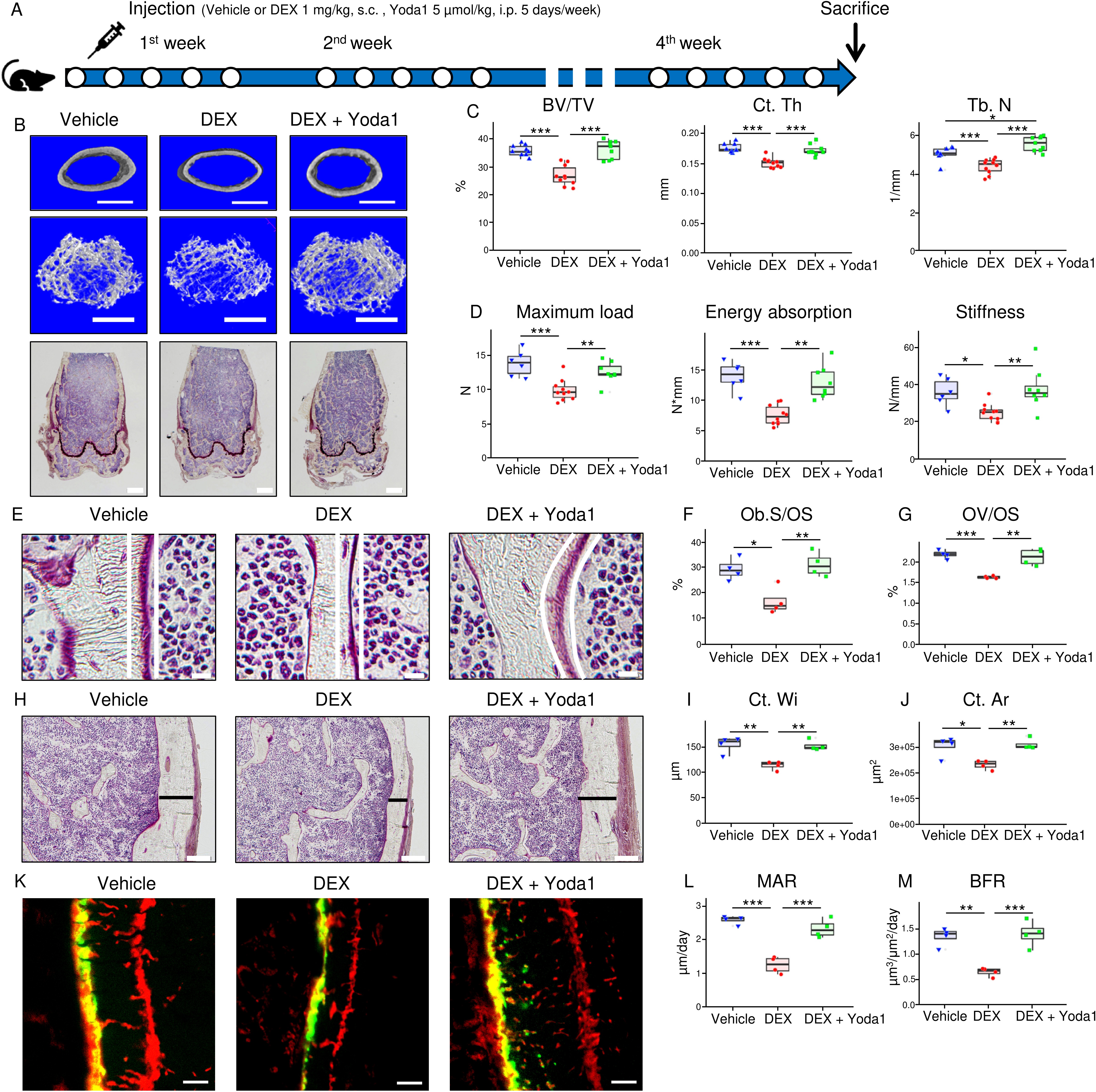
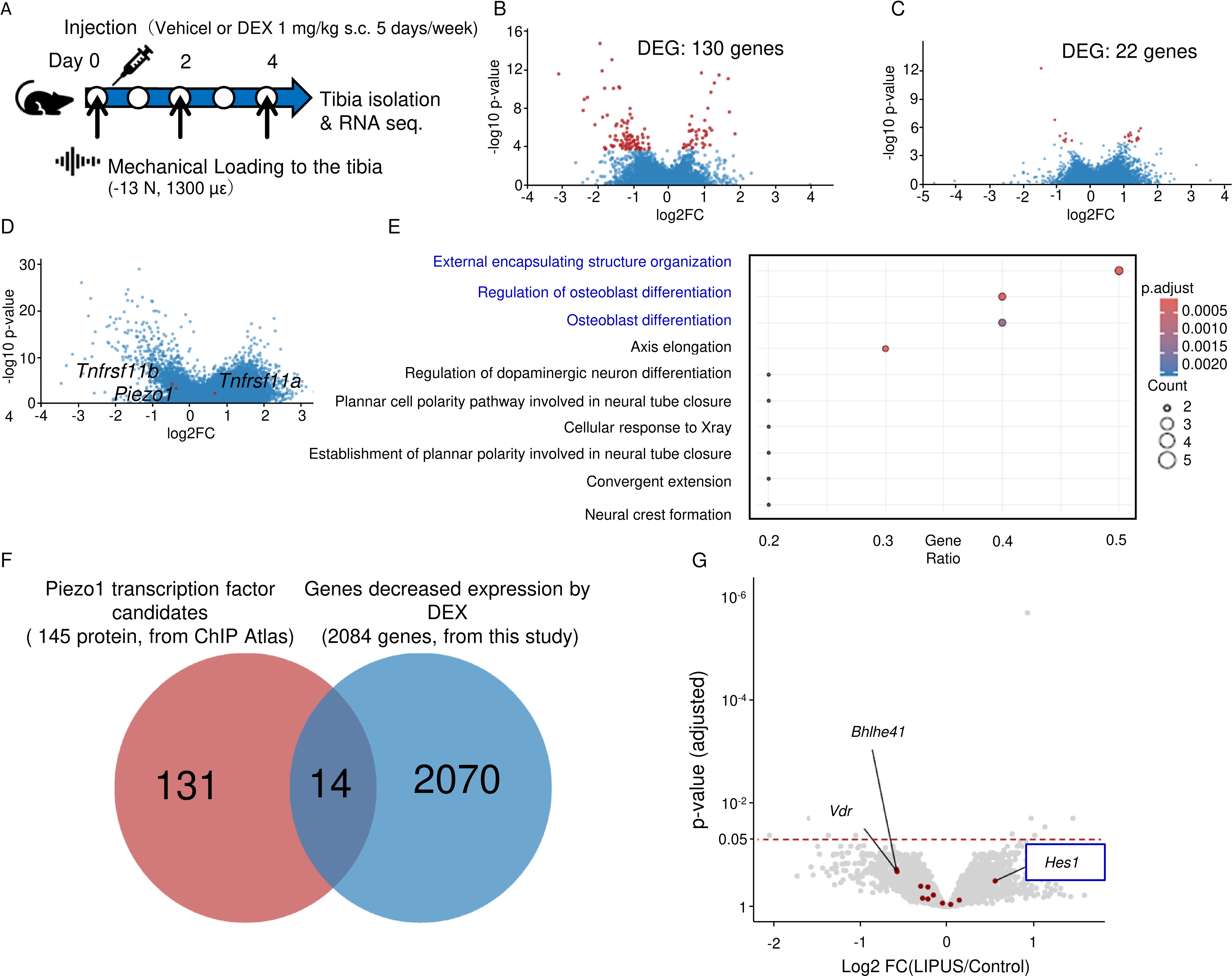
Results: GIOP patients demonstrated significantly reduced Piezo1 expression, compromised lacuno-canalicular network (LCN) integrity, and elevated apoptotic cell counts compared to controls. In the GIOP mouse model, DEX-induced reductions in bone mass, mechanical strength, and bone formation were substantially mitigated by Yoda1 treatment (Fig. 1). DEX significantly inhibited the increases in bone mass and formation attributable to mechanical stress in mouse tibiae. Cortical bone gene expression analyses revealed DEX-induced downregulation of Piezo1, the upregulation of the bone formation inhibitor sclerostin, and a higher bone resorption promoter RANKL/OPG ratio, which Yoda1 reversed. In MLO-Y4 cells, Yoda1 restored Piezo1 protein expression, intracellular Ca²⁺ influx, and subsequent phosphorylation of CaMkII, Akt, and ERK. RNA sequencing of mechanically loaded GIOP mouse bone tissue and ChIP atlas database analysis identified Hes1 as a novel Piezo1 transcription factor candidate (Fig. 2). Hes1 knockdown in MLO-Y4 cells markedly reduced Piezo1 expression, while CUT&RUN assays confirmed Hes1’s binding to the Piezo1 promoter region. Furthermore, luciferase assays verified Hes1’s role in promoting Piezo1 transcription. DEX suppressed, and Yoda1 enhanced, the expression and phosphorylation of Hes1 in MLO-Y4 cells.
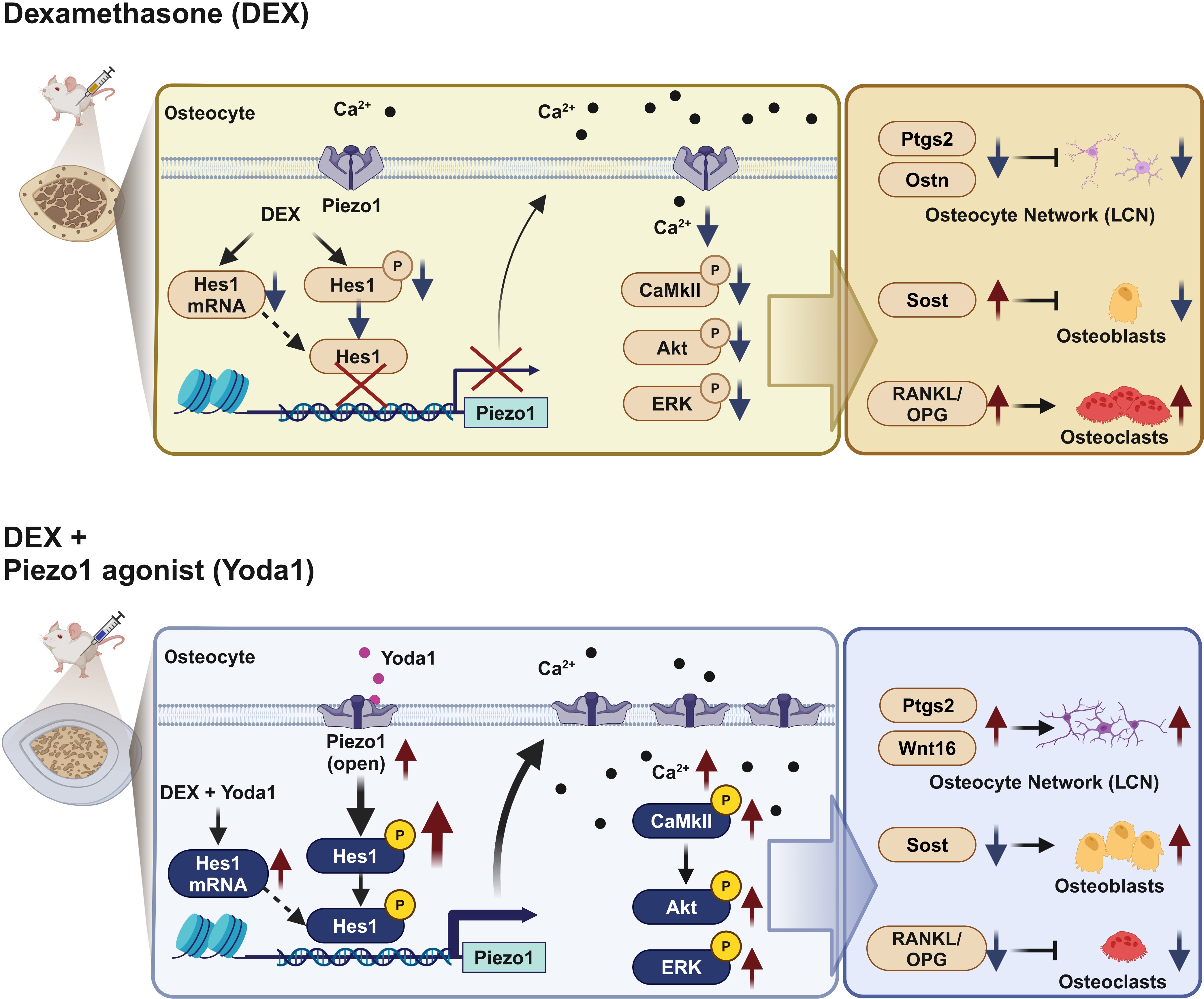
Conclusion: Our findings reveal a marked reduction in osteocytic Piezo1 expression in both GIOP patients and mouse models. DEX impeded Piezo1 expression by repressing the expression and phosphorylation of the transcription factor Hes1, thereby elevating sclerostin levels and the RANKL/OPG ratio. Conversely, Yoda1 augmented Hes1’s expression and phosphorylation, enhancing Piezo1’s transcriptional activity and expression, ultimately forestalling the bone mass and strength decline associated with GIOP (Fig. 3). These insights propose Piezo1 and its transcription factor Hes1 as novel therapeutic targets for GIOP mitigation.
To cite this abstract in AMA style:
Ochiai N, Etani Y, Noguchi T, Miura T, Kurihara T, Fukuda Y, Hamada H, Uemura K, Takashima K, Tamaki M, Ishibashi T, Ito S, Yamakawa S, Kanamoto T, Okada S, Nakata K, Ebina K. Activation of the Hes1/Piezo1 Pathway Promotes Mechanical Stress Response and Prevents Glucocorticoid-induced Osteoporosis [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/activation-of-the-hes1-piezo1-pathway-promotes-mechanical-stress-response-and-prevents-glucocorticoid-induced-osteoporosis/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/activation-of-the-hes1-piezo1-pathway-promotes-mechanical-stress-response-and-prevents-glucocorticoid-induced-osteoporosis/